Today students started with another math challenge to make sure they still remembered scientific notation and significant digits. It is Penguin Appreciation Day so students with 7/10 or better received a penguin sticker for their achievement.

We checked homework, review sheets, I answered questions, and students took ten minutes to cram before completing the Unit 1 Test. Students were quiet when they were finished to allow others to work. Highest score overall went to Lizzie and Kaboria. Congratulations! Here Joci, Jen, Kathy, and James are hard at work.
Because we had plenty of time and there was a high interest level in what chromatography entailed, I had gathered necessary materials for a easy quick demonstration. Directions were posted on the overhead on what to do (some read them, some did not) and materials were distributed. Pairs of students received a strip of filter paper and made a pencil line about 4 cm from the end. They then traced over the pencil line with a marker, dipped the paper into water and waited.

Chromatography is the separation of liquids based on particle or molecule size. Most inks are mixtures of colors and the ink will separate into bands of colors based on the size of the ink molecules.
In the photo you can see different group
s strips and the colors they separated into. In order, it was green, orange, black, blue, red, green, black. For example, the green marker separated into blue and yellow ink bands. The blue ink traveled faster and farther along the filter paper because it is made of smaller molecules. The yellow ink molecules did not travel as far or as fast because they are bigger.

The most interesting ink to separate is brown or black because they contain more colors (something I knew that they now know). This is Imani and Henry's black ink separating. Blue ink was the most boring, but surprisingly, red ink separated into a shade of pink and a shade of red. Trials with different types of markers may give different results.
Tomorrow we begin Unit 2 on Matter with new seats and new note packets.
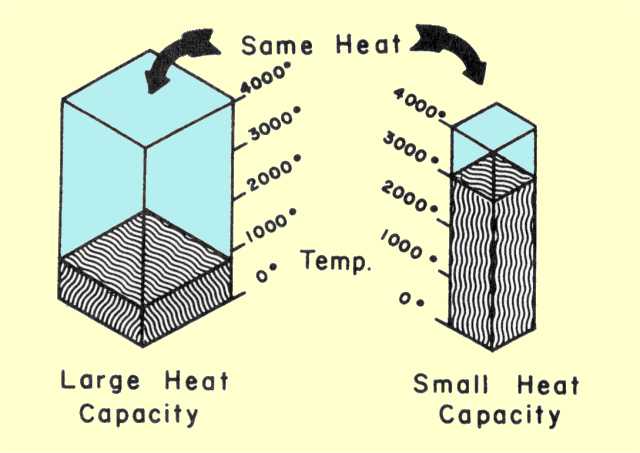
 We finished up notes by talking about boiling points, vapor pressure, and how heat changes all of those things. We are looking at a test Tuesday or Wednesday of next week.
We finished up notes by talking about boiling points, vapor pressure, and how heat changes all of those things. We are looking at a test Tuesday or Wednesday of next week. Students experimented with it and tried a variety of tasks including the hammer test, the burn test, pour and conformity and others. Rachael is trying the pour test and writing her results at the same time! She thinks this makes oobleck more like a liquid.
Students experimented with it and tried a variety of tasks including the hammer test, the burn test, pour and conformity and others. Rachael is trying the pour test and writing her results at the same time! She thinks this makes oobleck more like a liquid. Austin was very gung ho about trying the bounce test for his Oobleck. His conclusion is that oobleck bounces so it must be more like a solid. Oobleck even has its own Wikipedia article. For more information and more exact ways to make it, check out this article.
Austin was very gung ho about trying the bounce test for his Oobleck. His conclusion is that oobleck bounces so it must be more like a solid. Oobleck even has its own Wikipedia article. For more information and more exact ways to make it, check out this article.