Friday, January 30, 2015
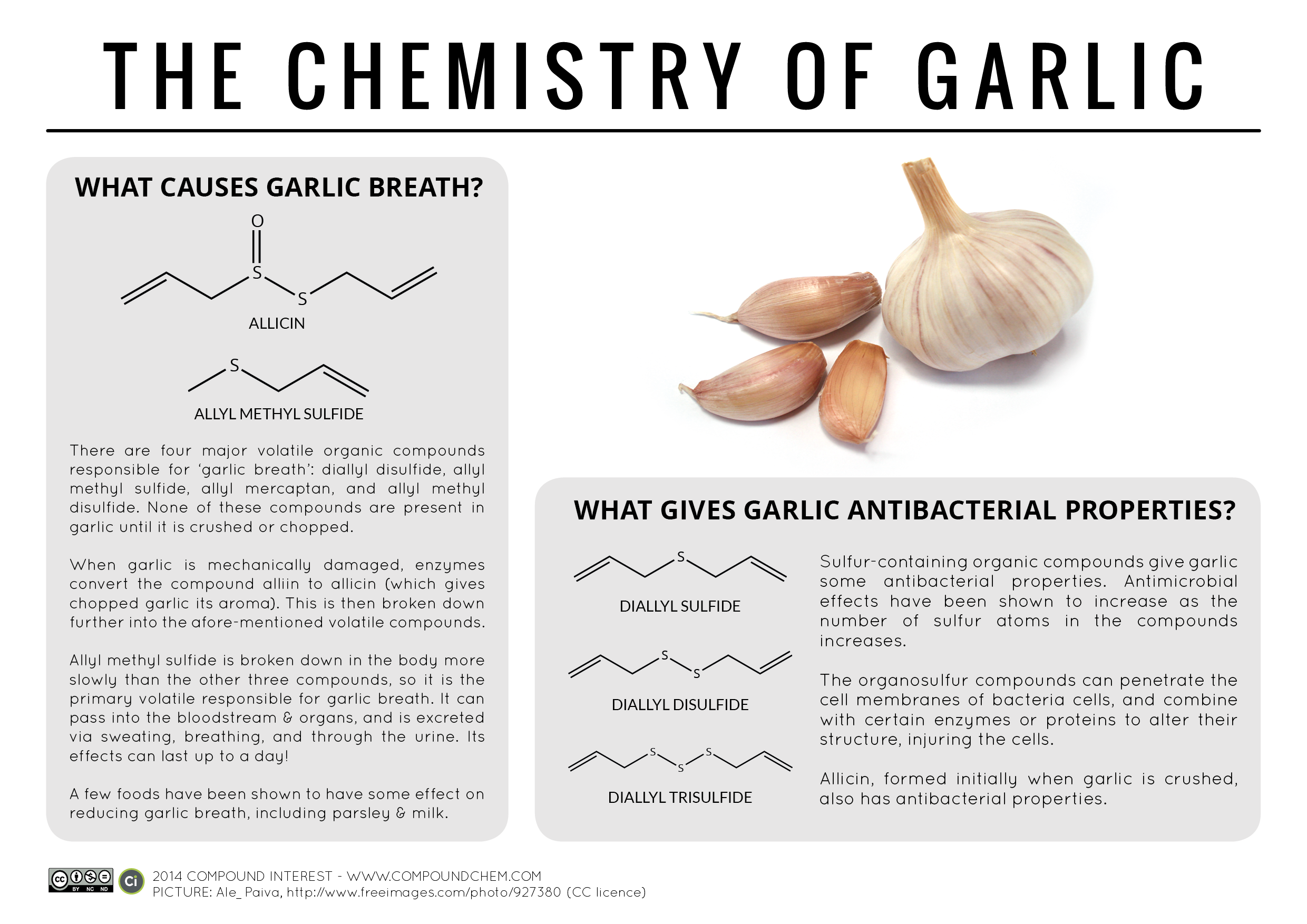
Chemistry Of: Body Odour and Garlic
Compound Interest's original post on Body Odour.
Compound Interest's original post on Garlic.
This TedEdTalk discusses why we pass gas (flatulence). Most human beings pass gas 10-20 times a day!
Grossed out that your skin is covered in billions or bacteria? Watch this TedTalk and "Meet your Microbes!"
In this TEDtalk, Lucy McRae hypothesizes that you could take a pill that would make you sweat perfume... among other body technological innovations.
In this TedTalk, Tristram Wyatt disusses human pheromones. Do our smells make us sexy?
Tuesday, January 27, 2015
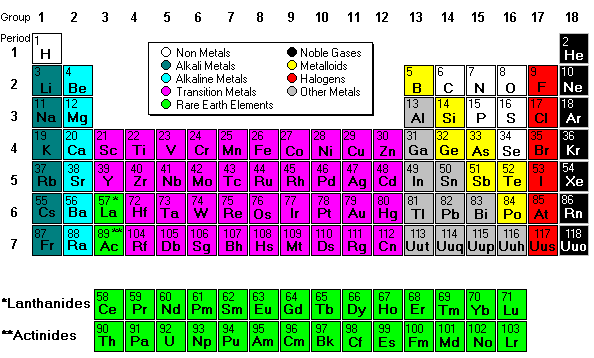
Dudes and the Periodic Table
There are several "dudes," famous chemistry folks that students need to be familiar with. These dudes (Democritus, Dalton, JJ Thompson, Millikan, Rutherford, Bohr, Heisenberg, De Broglie, and Planck) all did experiments and came up with different and improved atom models. The current model is the elctron cloud or quantum mechanical model which was formulated by Heisenberg and De Broglie.
Heisenberg and De Broglie came up with the current electron cloud model, but we draw Bohr's planetary model the most often because it easier to count the electrons. There's a TedEd video about the progression - HERE.
Electrons are tricky because they move constantly and at high speeds. Heisenberg's Uncertainty Principle states that you cannot know both the speed and location of an electron - you can only know one - because measuring either one, changes the other. DeBroglie's wave theory helps explain why electrons sometimes act like particles and sometimes are compared to waves.
For more information about the evolution of the atomic model, check out this link. And here is a video!
Here are some Dude Quizlet Flashcards to help you out.
Heisenberg and De Broglie came up with the current electron cloud model, but we draw Bohr's planetary model the most often because it easier to count the electrons. There's a TedEd video about the progression - HERE.
Electrons are tricky because they move constantly and at high speeds. Heisenberg's Uncertainty Principle states that you cannot know both the speed and location of an electron - you can only know one - because measuring either one, changes the other. DeBroglie's wave theory helps explain why electrons sometimes act like particles and sometimes are compared to waves.
For more information about the evolution of the atomic model, check out this link. And here is a video!
Here are some Dude Quizlet Flashcards to help you out.
Monday, January 26, 2015
Atoms and the Periodic Table
 Atoms, or elements, are the smallest unit of matter. They retain their identity in chemical reactions and are combined to form compounds and everything in the universe.
Atoms, or elements, are the smallest unit of matter. They retain their identity in chemical reactions and are combined to form compounds and everything in the universe.Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
How small is an atom? Watch this entertaining TedEd video!
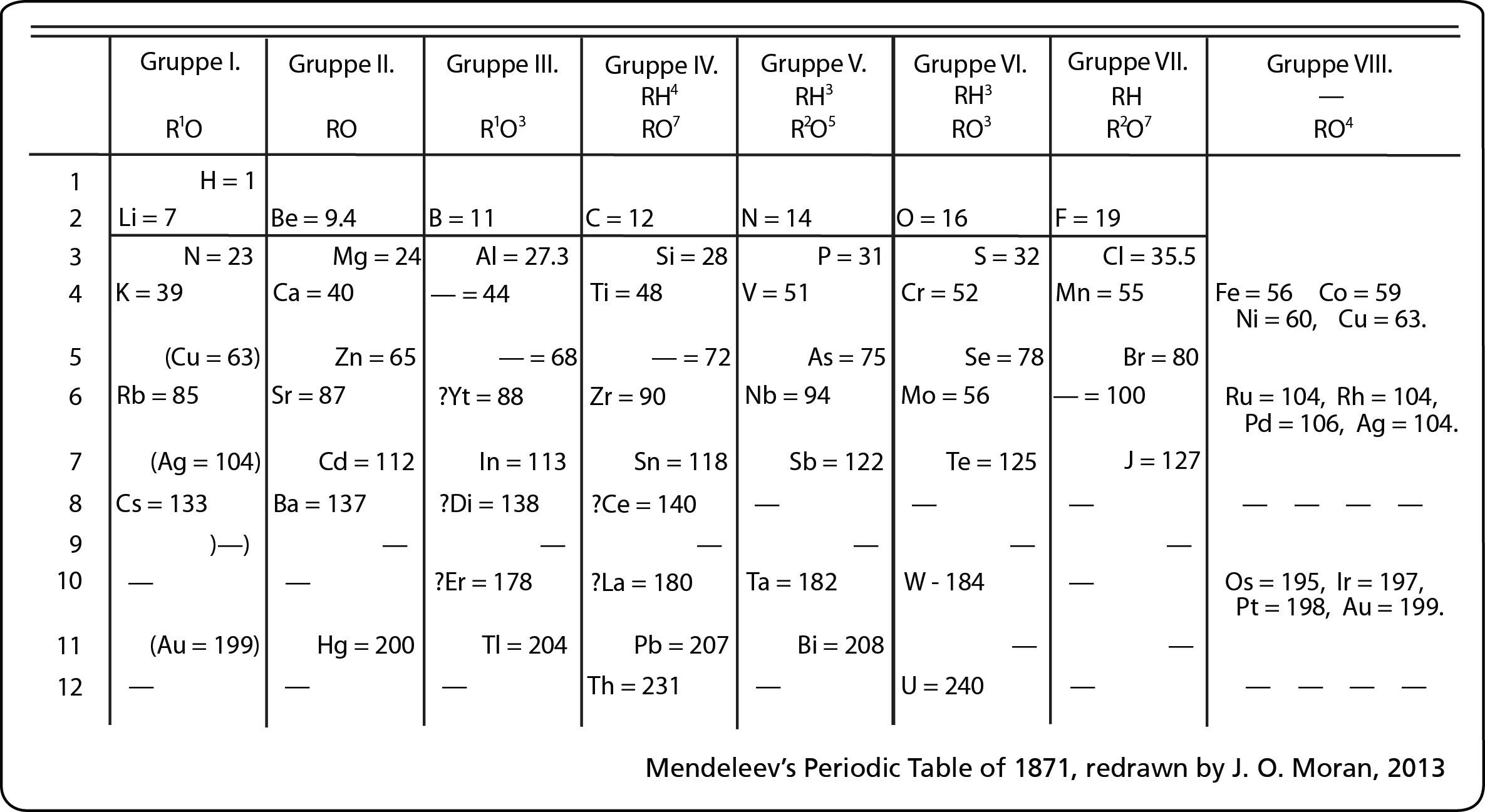 Mendeleev designed the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons). This is an AMAZING Video about Mendeleev and his PT!
Mendeleev designed the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons). This is an AMAZING Video about Mendeleev and his PT!Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
Friday, January 23, 2015
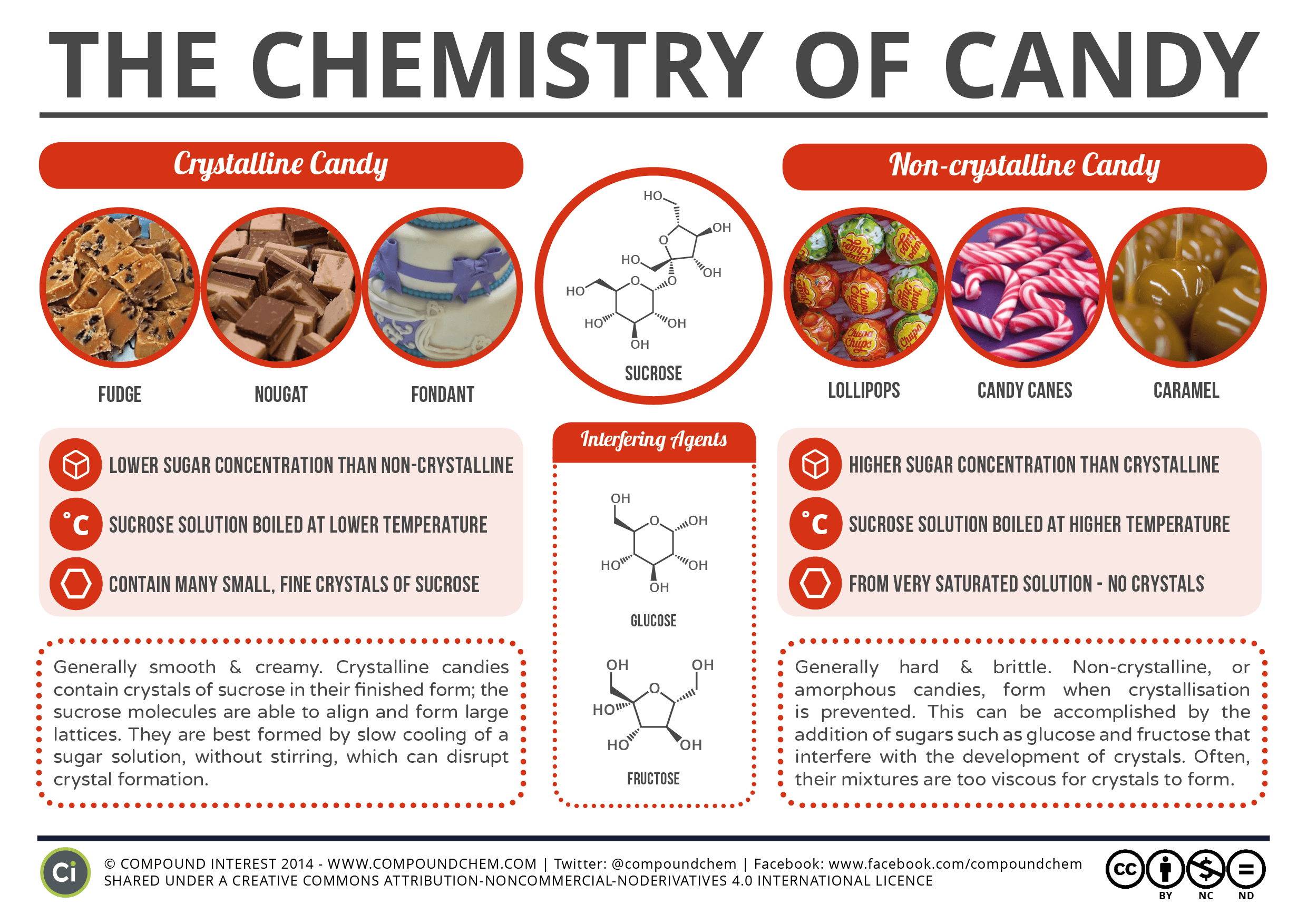
Chemistry of: Xylitol Gum and Candy
Compound Interest's original post on Xylitol Gum can be found here. Compound Interest's original post on the Chemistry of Candy can be found here.
For more information about how sugar is making its way into many of the foods you eat (and some that you least expect) check out this TedEd talk!
Some fun facts about candy from the American Chemical Society are
- Hard candy is technically a glass — so much so that it is sometimes used to make the “bottle” that gets broken over someone’s head in a fight scene.
- The same chemical that makes grapefruit taste sour — citric acid — is in sour-tasting candy.
- Peppermint oil comes from a plant, and some research shows that candies flavored with it can help people concentrate better.
- Ever wonder how they get liquefied cherries into a chocolate-covered cherry without leaving an injection hole? The candy actually starts with a hard cherry center that slowly softens after the chocolate is applied, thanks to a chemical called invertase that’s added to the recipe.
- Cotton candy is almost pure sugar that has been melted and then spun.
- Gummies contain flavor, sugar and a seaweed chemical called carrageenan, which makes them chewy.
- Licorice contains a smelly compound that’s found in a spice called anise.
 For more information about candy in the United States, check out the National Confectioner's Association.
For more information about candy in the United States, check out the National Confectioner's Association.
MsJ's favorite non-crystalline candy is probably the unlikely nerds rope. Mrs. Woodward's favorite candy is the cow tale.
Wednesday, January 21, 2015
Percent Composition and Bubble Gum Lab
Percent composition is just like determining your grade - the amount you got divided by the the whole amount.
With compounds, you find the mass of a particular element and divide it by the mass of the whole compound. So if you wanted to know the percent composition of oxygen in water, you would take the mass of oxygen and divide it by the mass of water.
We practiced some basics and the students measured the amount of sugar found in DubbleBubble bubble gum.
Students each had a piece of gum and observed the gum by weighing it, drawing it, and smelling it. The students chewed the gum for ten minutes. While they were waiting we watched How Its Made on bubblegum.
After ten minutes, students did more observations and re-weighed the gum. The gum weighed less... why? Because the sugar dissolved and was lost. Using this weight difference, students determined the percent composition of sugar in the gum they chewed. They also can convert the grams to moles and determine how many moles of sugar were in the gum.
With compounds, you find the mass of a particular element and divide it by the mass of the whole compound. So if you wanted to know the percent composition of oxygen in water, you would take the mass of oxygen and divide it by the mass of water.
We practiced some basics and the students measured the amount of sugar found in DubbleBubble bubble gum.
Students each had a piece of gum and observed the gum by weighing it, drawing it, and smelling it. The students chewed the gum for ten minutes. While they were waiting we watched How Its Made on bubblegum.
After ten minutes, students did more observations and re-weighed the gum. The gum weighed less... why? Because the sugar dissolved and was lost. Using this weight difference, students determined the percent composition of sugar in the gum they chewed. They also can convert the grams to moles and determine how many moles of sugar were in the gum.
Monday, January 19, 2015
What is a mole?
Moles are used to count atoms. There are 22,000,000,000,000,000,000 quintillion atoms in a grain of sand and even counting grains of sand is a pain. Because atoms are so tiny, we use the mole to estimate.
There are 6.02 x 10 ^23 molecules in one mole. That's a whole lot. This is our new favorite number because it needs to be memorized. We will practice converting from moles to molecules.
Next we discussed molar mass. Molar mass = 1 mole and it also equals atomic mass from the periodic table. To find the molar mass of carbon dioxide you find the mass of carbon and two oxygens and add them together. Finding molar mass is not difficult unless the molecule has tricky subscripts (which we have been practicing).
The third thing about moles is that "one mole of any gas will occupy 22.4 Liters." 22.4 is another favorite number. We can convert from moles to liters and from liters to moles.
Just how big is a mole? There's a TedEd talk on that! Watch it here!
There are 6.02 x 10 ^23 molecules in one mole. That's a whole lot. This is our new favorite number because it needs to be memorized. We will practice converting from moles to molecules.
Next we discussed molar mass. Molar mass = 1 mole and it also equals atomic mass from the periodic table. To find the molar mass of carbon dioxide you find the mass of carbon and two oxygens and add them together. Finding molar mass is not difficult unless the molecule has tricky subscripts (which we have been practicing).
The third thing about moles is that "one mole of any gas will occupy 22.4 Liters." 22.4 is another favorite number. We can convert from moles to liters and from liters to moles.
Just how big is a mole? There's a TedEd talk on that! Watch it here!
Friday, January 16, 2015
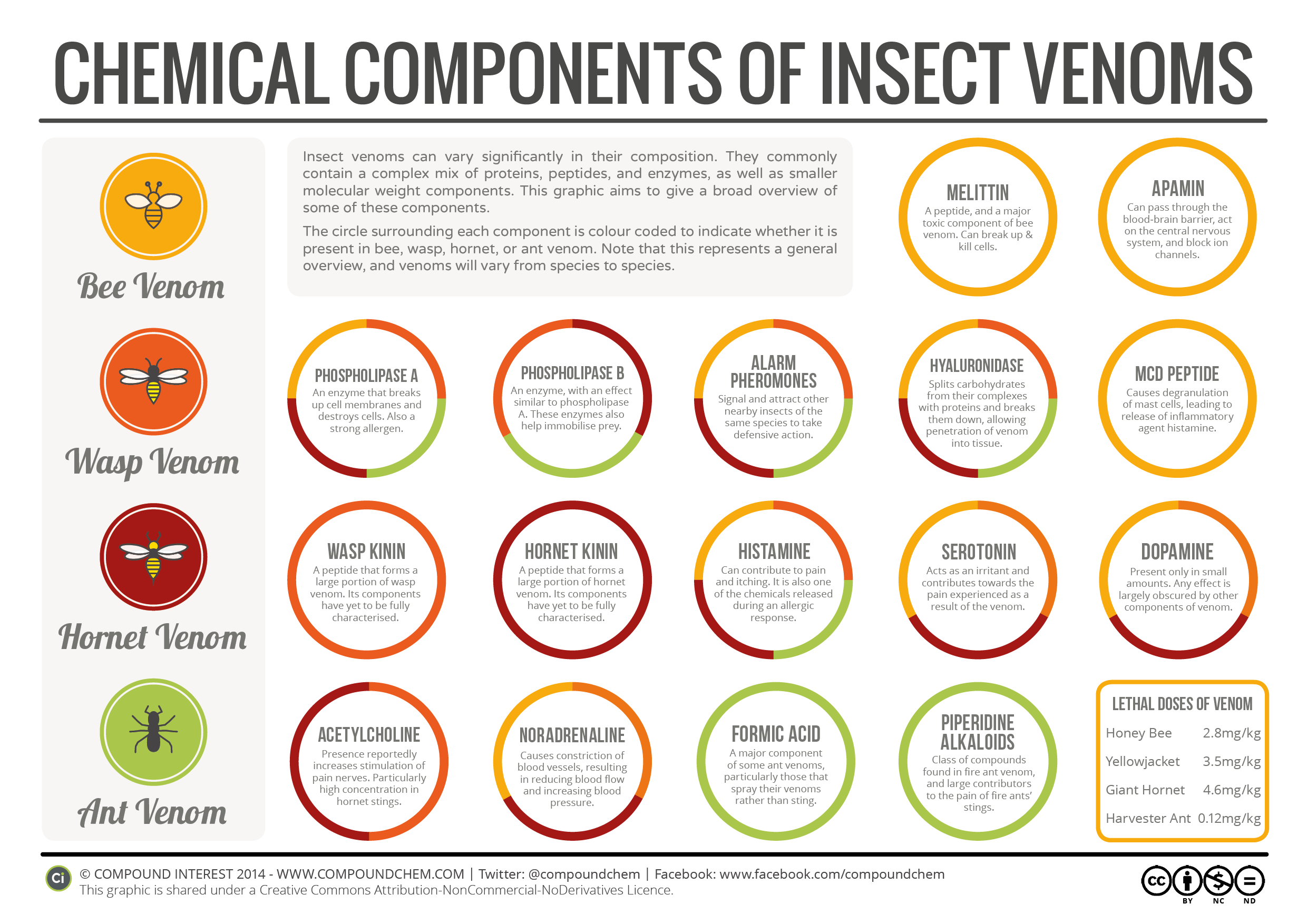
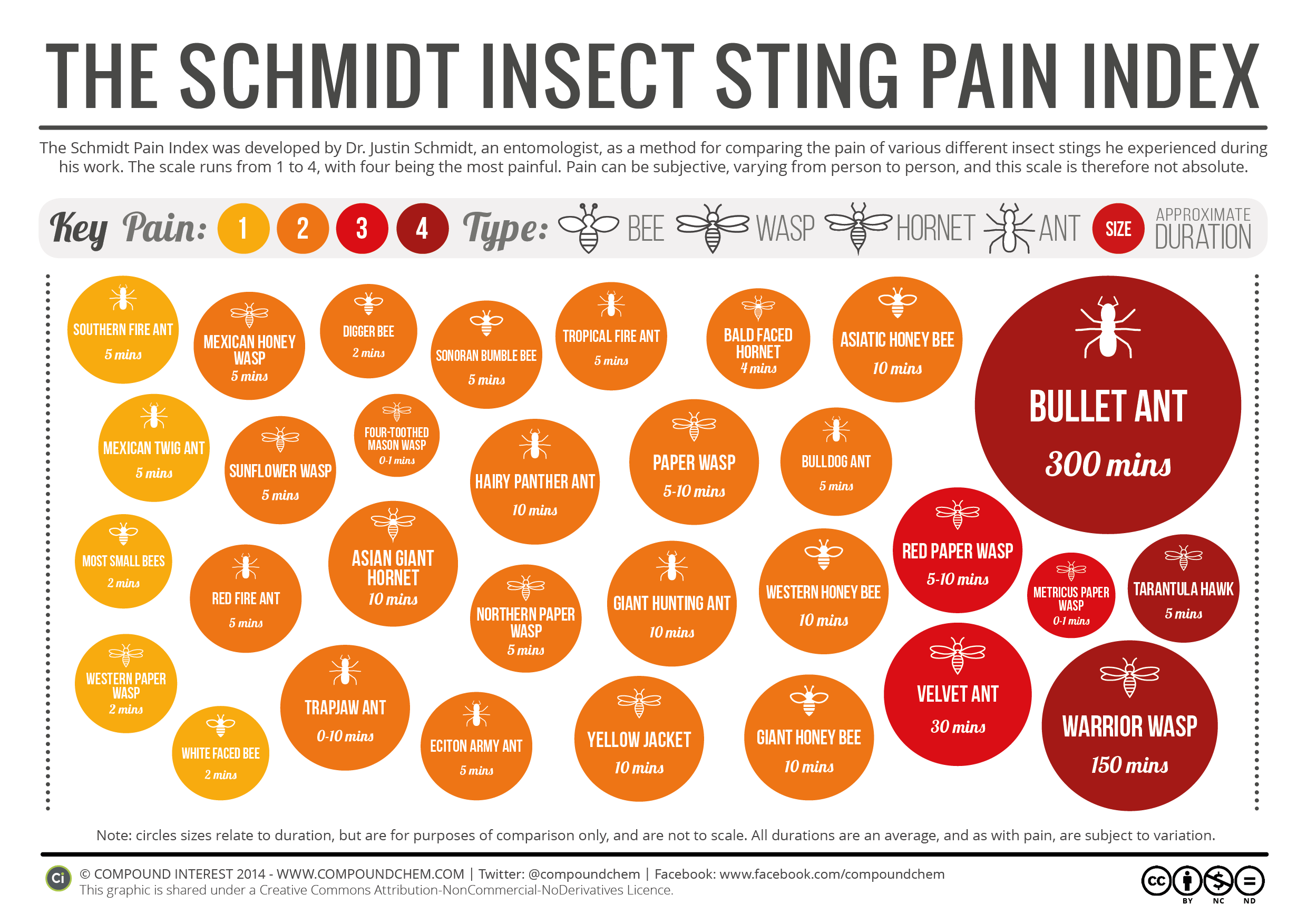
Chemistry Of: Insect Venom
You can listen to an interview with Schmidt who developed the pain scale here (it is the first ten minutes) on a RadioLab podcast (I highly recommend their shows).
The Velvet Ant found in Virginia is red and black and therefore relatively easy to avoid. Velvet ants are actually female wingless wasps. More information about it can be found here.
What's the difference between poison and venom? There's a TedEd Talk on that! Watch it HERE.
How does your brain respond to pain? There's a TedEd Talk on that! Watch it HERE!
Want to know more about ant colonies? There's a TED talk on it. Watch it here.
The original Compound Interest article on insect venom and the Schmidt pain index can be found here.
Thursday, January 15, 2015
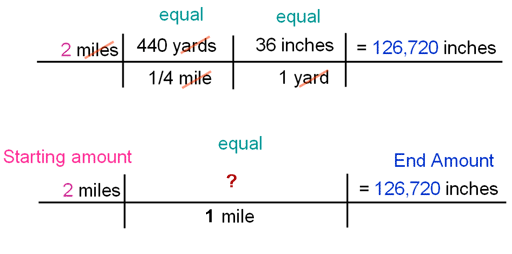
Conversions (Dimensional Analysis)
Conversions, or dimensional analysis, are used to change one unit to another. This is really useful when converting to metric units, but is essential to chemistry in terms of mole conversions. Setting up conversions is a skill so we started with learning the format. There are plenty of how to videos out there on the internet for anyone needing a tutorial.

We practiced conversions with some of the crazy things people do to get in the Guinness Book of World Records - like longest ear hair, skinniest waist, tallest man, etc. I think the one the kids thought was the weirdest was the lady who can pop her eyes out 12mm.
Check out more crazy records here!

We practiced conversions with some of the crazy things people do to get in the Guinness Book of World Records - like longest ear hair, skinniest waist, tallest man, etc. I think the one the kids thought was the weirdest was the lady who can pop her eyes out 12mm.
Check out more crazy records here!
Monday, January 12, 2015
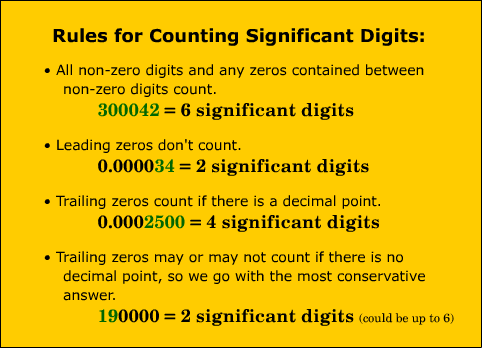
Significant digits and Scientific Notation
Significant digits are used so that our calculations are not more precise than our measurements. All digits other than zero are significant.
Its the zeros that are the tricky ones. We went over the rules and then did some practice situations. We will continue to practice this and all calculations made for the rest of the year must be rounded to the correct number of significant digits.
Scientific Notation is used for very large and very small numbers that usually have a lot of zeros to make the numbers for manageable. Most students are familiar with scientific notation and just need a bit of practice.
Its the zeros that are the tricky ones. We went over the rules and then did some practice situations. We will continue to practice this and all calculations made for the rest of the year must be rounded to the correct number of significant digits.
Scientific Notation is used for very large and very small numbers that usually have a lot of zeros to make the numbers for manageable. Most students are familiar with scientific notation and just need a bit of practice.
Friday, January 9, 2015
Chemistry Of: Lethal Doses and Natural vs. ManMade Chemicals
This week's topic is about lethal doses and LD50.
The second portion is about the difference in toxicity of natural vs. manmade chemicals. Most people wrongly assume that if a chemical is natural that is good and that if a chemical is manmade then it is bad. Both can be good and both can be bad!
Compound Interest's original post about Lethal Doses of Common Chemicals can be found here.
Compound Interest's original post about Natural vs. ManMade Chemicals can be found here.
The second portion is about the difference in toxicity of natural vs. manmade chemicals. Most people wrongly assume that if a chemical is natural that is good and that if a chemical is manmade then it is bad. Both can be good and both can be bad!
Compound Interest's original post about Lethal Doses of Common Chemicals can be found here.
Compound Interest's original post about Natural vs. ManMade Chemicals can be found here.
Thursday, January 8, 2015
Chromatography Lab
Chromatography is the separation of liquids based on particle or molecule size.
Chromatography is used to separate inks into the colors that make them up. Most inks are mixtures of colors and the ink will separate into bands of colors based on the size of the different ink molecules.The small particles move faster and will move further up the paper strip (in the photo black); the larger particles do not move as far (yellow). Some black inks are actually made up of blue red, pink, and yellow.
Pairs or groups of students received a strip of filter paper and made a pencil line about 3 cm from the end. They then traced over the pencil line with a marker, dipped the edge of the paper into water and waited. Over time the water will travel through the paper carrying the ink molecules with it. Smaller molecules are easier to carry and travel further.
 Chromatography can be used to separate any mixtures with different size particles and actually gel electrophoresis used for DNA analysis works on a similar principle.
Chromatography can be used to separate any mixtures with different size particles and actually gel electrophoresis used for DNA analysis works on a similar principle.
Chromatography is used to separate inks into the colors that make them up. Most inks are mixtures of colors and the ink will separate into bands of colors based on the size of the different ink molecules.The small particles move faster and will move further up the paper strip (in the photo black); the larger particles do not move as far (yellow). Some black inks are actually made up of blue red, pink, and yellow.
Pairs or groups of students received a strip of filter paper and made a pencil line about 3 cm from the end. They then traced over the pencil line with a marker, dipped the edge of the paper into water and waited. Over time the water will travel through the paper carrying the ink molecules with it. Smaller molecules are easier to carry and travel further.
 Chromatography can be used to separate any mixtures with different size particles and actually gel electrophoresis used for DNA analysis works on a similar principle.
Chromatography can be used to separate any mixtures with different size particles and actually gel electrophoresis used for DNA analysis works on a similar principle.Wednesday, January 7, 2015
NFPA Hazard Diamonds
Most of us have seen these diamonds on trucks as we pass them on the highway. These diamonds are useful for safety and response teams to identify what is in the truck so the correct precautionary and clean up measures can be made.
The NFPA 704 fire diamond (or hazmat diamond) is described in NFPA Standard 704, maintained by the National Fire Protection Association. The system identifies four key hazards (health (blue), flammability (red), instability (yellow), and special (white)) and their degree of severity. Hazard severity is rated numerically, ranging from 0 (minimal) to 4 (severe).
Want to make your own customizable hazard diamond? Click here. Why does this feature exist? So people can make easily identifiable hazard diamonds for any chemical they have on hand.
Tuesday, January 6, 2015
Lab Equipment Identification
 Students need to be familiar with certain lab equipment and its purpose, even if we won't use all of it in labs for high school chemistry. Students identified equipment using examples of the real thing (we made this into a contest) and also by identifying black and white drawings.
Students need to be familiar with certain lab equipment and its purpose, even if we won't use all of it in labs for high school chemistry. Students identified equipment using examples of the real thing (we made this into a contest) and also by identifying black and white drawings.Students will sort equipment based on what its main purpose is. Some equipment is used for liquids, some for solids, and some for heating. Some is used for all of those things.
The three pieces of equipment most likely to come up, and the most confusing when in black and white photos are the watch glass, evaporating dish, and the crucible (which has a lid). All three of these can be used for heating substances to remove water among other things. In real life they look different - but in drawings they look similar. How to tell them apart? The watch glass resembles a contact lense; the evaporating dish has a pour spout; and the crucible is more cup shaped and often is pictured with a lid.
Here is an online quiz on lab equipment with photos! Try it!
Friday, January 2, 2015
Welcome Spring 2015
Greetings students, parents, and guardians.
Welcome to a new school year with Ms Jancaitis! This blog has been set up to connect students, parents, and guardians with the chemistry class.
Each student will receive a course syllabus. The course syllabus outlines what the course will be like, what topics will be covered, and course expectations. It also contains contact information. There will be a quiz on this syllabus on Thursday.
Our first unit will cover lab safety and equipment. Each note packet comes with the safety rules and contract. The safety rules and contract need to be read and signed by both the student and parent guardian. The safety rules are rules designed to keep the classroom safe and orderly to maximize learning and prevent accidents and injuries. These rules need to be studied because there will be a safety test on Monday or Tuesday and infractions of these rules can lead to disciplinary action as well as low assignment grades. A contract holds students accountable for the items that are broken if the student is acting a manner that is unsafe for themselves or those around them.
Please have these papers signed and returned by Monday the 12th. Students not returning signed safety rules and safety contracts will not be able to participate in labs and activities until the contracts are signed and returned.
Welcome to a new school year with Ms Jancaitis! This blog has been set up to connect students, parents, and guardians with the chemistry class.
Each student will receive a course syllabus. The course syllabus outlines what the course will be like, what topics will be covered, and course expectations. It also contains contact information. There will be a quiz on this syllabus on Thursday.
Our first unit will cover lab safety and equipment. Each note packet comes with the safety rules and contract. The safety rules and contract need to be read and signed by both the student and parent guardian. The safety rules are rules designed to keep the classroom safe and orderly to maximize learning and prevent accidents and injuries. These rules need to be studied because there will be a safety test on Monday or Tuesday and infractions of these rules can lead to disciplinary action as well as low assignment grades. A contract holds students accountable for the items that are broken if the student is acting a manner that is unsafe for themselves or those around them.
Please have these papers signed and returned by Monday the 12th. Students not returning signed safety rules and safety contracts will not be able to participate in labs and activities until the contracts are signed and returned.
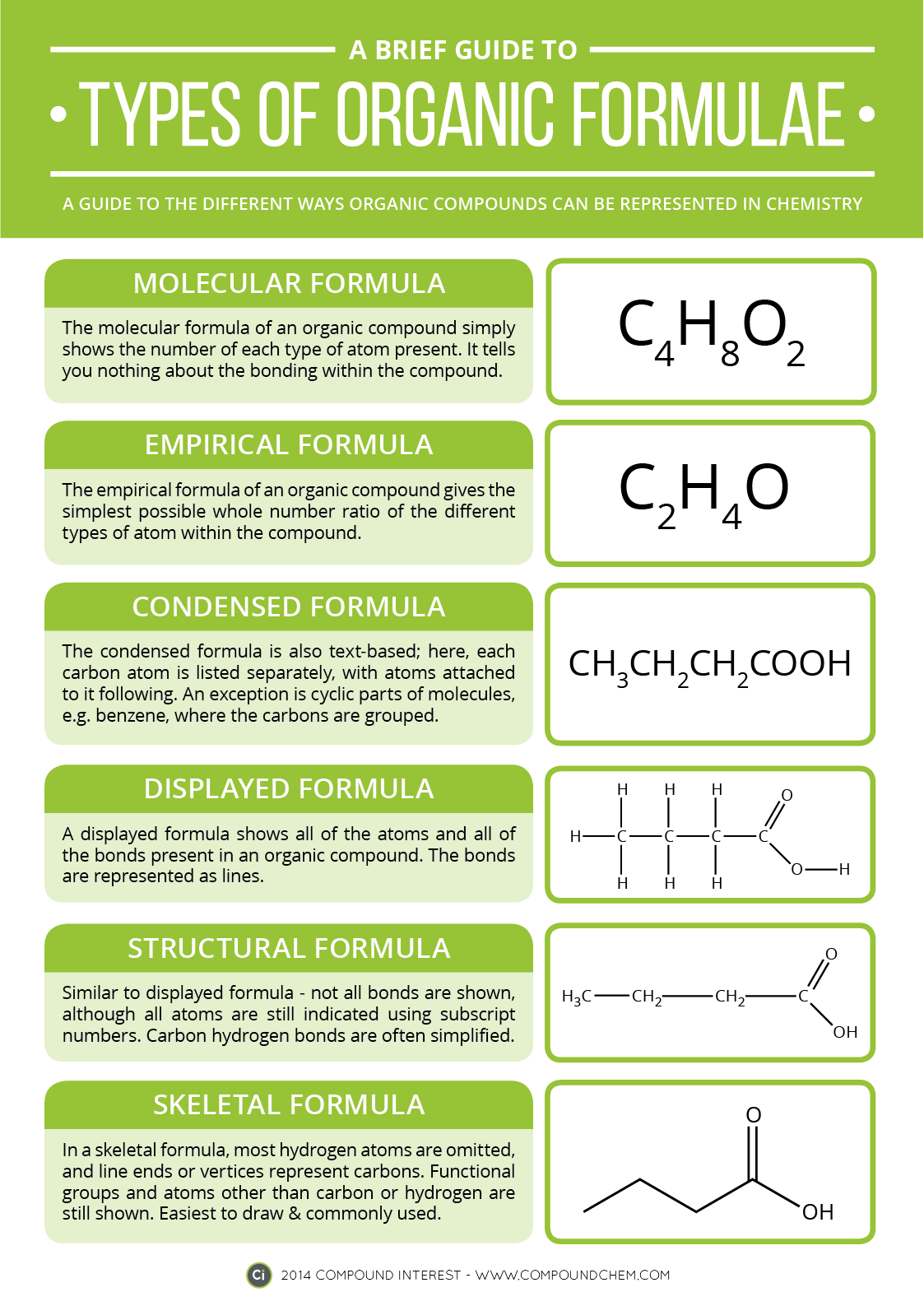
Chemistry Of: Types of Organic Formulas
Each week you will need to complete a "Chemistry of" assignment based on infographics pertaining to real life chemistry. Here is the first assignment... information that will be helpful for decoding chemistry for the rest of the semester. Click on the image to make it larger.
Compound Interest's original post can be found here.