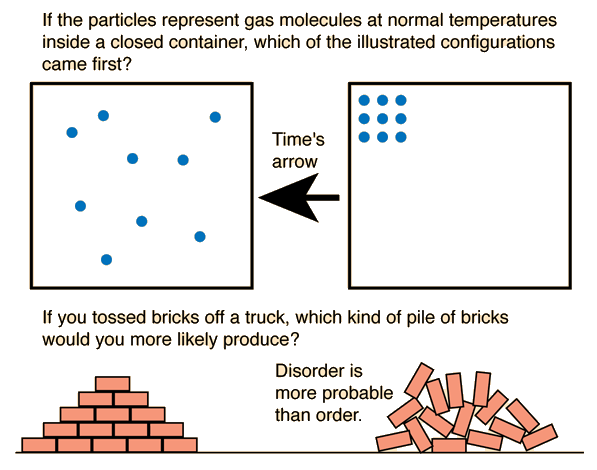
Entropy
is a chemistry word for disorder. An increase in entropy is
spontaneous. By looking for four things in a reaction, students can
determine whether a reaction is spontaneous or nonspontaneous by looking
for an increase in entropy.
Exothermic reactions are spontaneous and show an increase in entropy.
Gases are messier than solids, so a reaction that forms a gas shows an increase in entropy.
More
molecules show an increase in entropy. Count the coefficients on either
side of a balanced equation. If the products have more molecules then
there is an increase in entropy and the reaction could be spontaneous.
A decrease in the size of molecules (count atoms making up the molecule) is an increase in entropy.
Students
look for all four things and decide whether the overall reaction would
lead to an increase in entropy and be spontaneous.
Monday, October 17, 2016
Friday, October 14, 2016
Physical vs. Chemical
We talked about the Law of Conservation of mass and how matter cannot be created or destroyed. If you burn a log, the mass of all the ashes, smoke, gases, and everything that is burned off and left behind EQUALS the mass of the original log.
Today students discussed physical vs. chemical properties and changes. They've heard all of this before I am sure, but it doesn't hurt to go over it again. Then we did a challenge to see if they really knew their stuff.
Need to practice identifying chemical and physical properties? Check this out!
Need help identifying types of matter and whether they are heterogeneous or homogeneous? Check this out!
Here's a helpful video lecture --> HERE
Thursday, October 13, 2016
Le Chatlier - shifting equilbrium to reduce stress
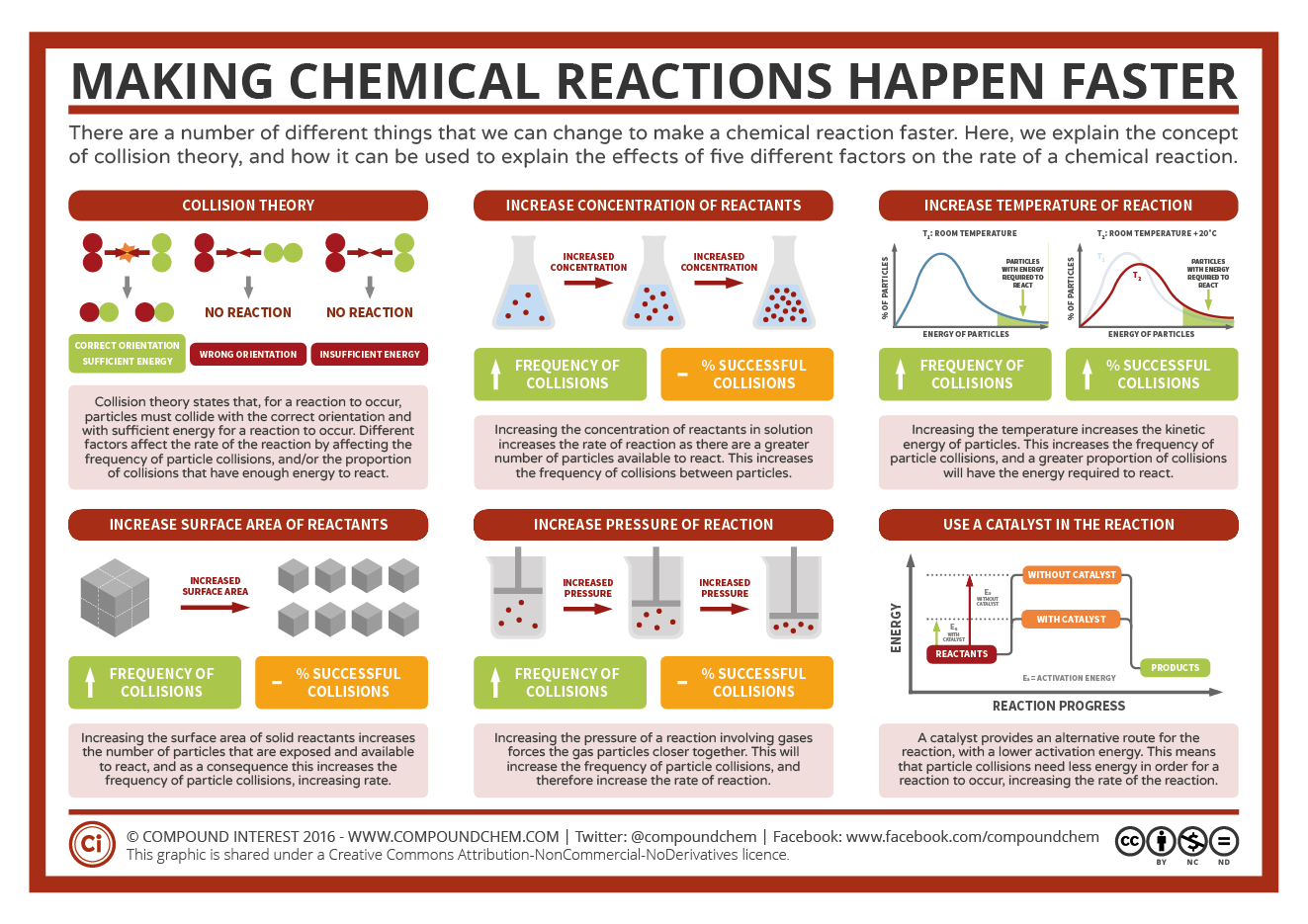
Students learned about reaction rates and how to increase them. They
also learned about reversible reactions and how Le Chatlier's principle
influences shifts of equilibrium in reversible reactions.
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
 Pressure
is the tricky one. If pressure is applied to an equilibrium, then the
reaction will shift to the side that has the least amount of molecules
(count the coefficients).
Pressure
is the tricky one. If pressure is applied to an equilibrium, then the
reaction will shift to the side that has the least amount of molecules
(count the coefficients).
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
 Pressure
is the tricky one. If pressure is applied to an equilibrium, then the
reaction will shift to the side that has the least amount of molecules
(count the coefficients).
Pressure
is the tricky one. If pressure is applied to an equilibrium, then the
reaction will shift to the side that has the least amount of molecules
(count the coefficients).Wednesday, October 12, 2016
Reaction Rate Basics
 Reaction
Rates are affected by a few things. Without telling them the point, the
students had a quick demo where they had to dissolve sugar cubes the
fastest. The things that speed up reactions are:
Reaction
Rates are affected by a few things. Without telling them the point, the
students had a quick demo where they had to dissolve sugar cubes the
fastest. The things that speed up reactions are:- Temperature - warmer is faster
- Surface Area - small pieces have more surface area
- Concentration - the more water, the faster sugar will dissolve
- Catalyst - lowers the activation energy and speeds up the reaction
- Agitation - shaking or stirring increases the frequency of collisions
Tuesday, October 11, 2016
Reaction Types
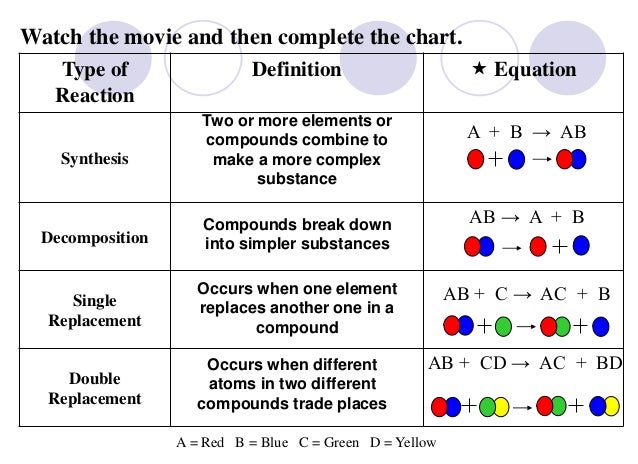 We
started by talking about the simple definition of the terms, what the
probably products and reactants are and went over a basic formula for
the reaction types the students need to be familiar with.
We
started by talking about the simple definition of the terms, what the
probably products and reactants are and went over a basic formula for
the reaction types the students need to be familiar with.Reaction Types include:
- synthesis
- decomposition
- singe replacement
- double replacement
- combustion
- endothermic
- exothermic
- oxidation-reduction
- neutralization
- nuclear
Can you guess what type this is?
Friday, October 7, 2016
Balancing Reactions
Students
are learning to balance equations. Today they learned that reactants
are what you start with and are on the left side of the equation.
Products are on the right side of the arrow and are what is made by
process of a chemical change.
Because of the Law of Conservation of Mass, the number of atoms have to be equal on both sides. To balance an equation, the coefficients are changed. Coefficients are the big numbers in front that tell you how many molecules there are. The subscripts (the little lower numbers) are not allowed to be changed because those are there to make neutrally bonded molecules (what we learned in the last unit.
By changing the coefficients and counting the number of atoms on both sides of the arrow, balancing can be achieved.
Because of the Law of Conservation of Mass, the number of atoms have to be equal on both sides. To balance an equation, the coefficients are changed. Coefficients are the big numbers in front that tell you how many molecules there are. The subscripts (the little lower numbers) are not allowed to be changed because those are there to make neutrally bonded molecules (what we learned in the last unit.
By changing the coefficients and counting the number of atoms on both sides of the arrow, balancing can be achieved.