Today after a jump in and going over last nite's homework, students learned about ionic bonding. Ionic bonding happens between metals & nonmetals (positives & negatives). After learning the basics, students practiced with an activity called "speed dating." Students were metals (boys) and nonmetals (girls) and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. Pictured are Zack (lead +4) and Sam (carbon -4) on their date.
Homework = Front of Counting Atoms Worksheet
Wednesday, September 22, 2010
Monday, September 20, 2010
Valence Electrons
Today students learned about valence electrons. Valence electrons are the outermost electrons and are the electrons that are used for bonding and participate in reactions. Valence electrons are only found in the S and P blocks.
Students practiced counting valence electrons and drawing Lewis Dot Structures.
Students also learned how to identify the charges of metals with more than one oxidation state using Roman numerals.
Homework - Box H on gold sheet
Students practiced counting valence electrons and drawing Lewis Dot Structures.
Students also learned how to identify the charges of metals with more than one oxidation state using Roman numerals.
Homework - Box H on gold sheet
Wednesday, September 15, 2010
Finishing up the Periodic Table
Students started the day with a foldable reviewing all the dudes they need to know that influenced the develop- ment of the atomic model. Students tried it out without their notes and realized how little they remembered... and then used their notes to record the information. We also watched a BrainPop about the atomic models to help refresh their memories. Now that they have their foldables made, they can use them to study for Friday's unit test and to study for the SOL.
Next we finished up the notes by discussing periodic trends.
Electro- negativity is how badly atoms want electrons. The most electronegative atoms are Fluorine, Chlorine, and Oxygen. Ionization energy is how difficult it is to remove electrons. It is difficult to remove electrons from atoms that are electronegative.
Atomic radius increases as you move down the periodic table because atoms have more mass, but actually decreases from left to right because atoms are holding on to their electrons tighter (because they are more electronegative).
To finish class students worked on a review sheet for Friday's unit test.
Homework tonight - work on packets and Box J on the Gold Sheet.
Next we finished up the notes by discussing periodic trends.
Electro- negativity is how badly atoms want electrons. The most electronegative atoms are Fluorine, Chlorine, and Oxygen. Ionization energy is how difficult it is to remove electrons. It is difficult to remove electrons from atoms that are electronegative.
Atomic radius increases as you move down the periodic table because atoms have more mass, but actually decreases from left to right because atoms are holding on to their electrons tighter (because they are more electronegative).
To finish class students worked on a review sheet for Friday's unit test.
Homework tonight - work on packets and Box J on the Gold Sheet.
Tuesday, September 14, 2010
Benchmark
Today students practiced electronic configurations. They did an excellent job of trying to figure it out and worked quietly on things while I got around to help people that had questions.
Students then took their first benchmark to assess their progress. Students performed well and are scoring where they should.
Students finished class by working on a periodic table worksheet and working on review materials. The Unit 3 Test will be at the end of the week.
Homework - finish periodic table worksheet (it has a question mark on it) and boxes D and F on gold sheet.
Students then took their first benchmark to assess their progress. Students performed well and are scoring where they should.
Students finished class by working on a periodic table worksheet and working on review materials. The Unit 3 Test will be at the end of the week.
Homework - finish periodic table worksheet (it has a question mark on it) and boxes D and F on gold sheet.
Monday, September 13, 2010
Electronic Configuration and Battleship
Today students learned the pattern of electronic configuration and how to use it. Basically its like giving directions to an element on the PT using set landmarks. It is a bit confusing, but once you get the pattern, its not too bad. We practiced with SPDF and electron configuration with arrows.
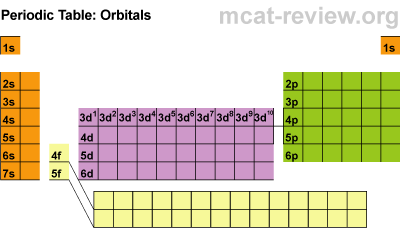
 Students learned about orbitals of the periodic table. These are SPDF. The letters have to do with the shapes the electrons travel around the nucleus in the electron cloud.
Students learned about orbitals of the periodic table. These are SPDF. The letters have to do with the shapes the electrons travel around the nucleus in the electron cloud.
Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.
I am not that good at Battle ship... but in fourth I played two separate games against Josh and Hannah... I think they both hit more of my ships than I hit of theirs.
 Students learned about orbitals of the periodic table. These are SPDF. The letters have to do with the shapes the electrons travel around the nucleus in the electron cloud.
Students learned about orbitals of the periodic table. These are SPDF. The letters have to do with the shapes the electrons travel around the nucleus in the electron cloud. Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.
 |
| Tessa and Andrew go head to head with Electronic Configuration Battleship |
Friday, September 10, 2010
Electronic Configuration
Students started class with a BrainPop and a worksheet related to Atoms. Scores were very high in all three portions of the competition.
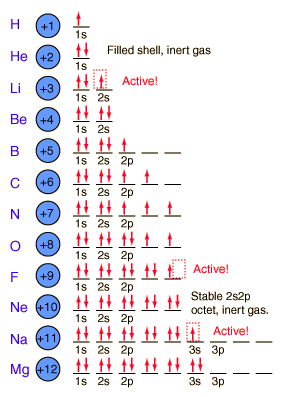
Today in class students learned about electron orbitals. First students learned how many electrons go on the electron shells for a Bohr model.
2 can go on the first ring (corresponding with the 2 elements on the first period of the periodic table). If you look at all the models to the left, they all only have 2 electrons on the first ring near the nucleus. 8 go on the 2nd ring, just like there are 8 elements in the 2nd period. The next ring gets 18.
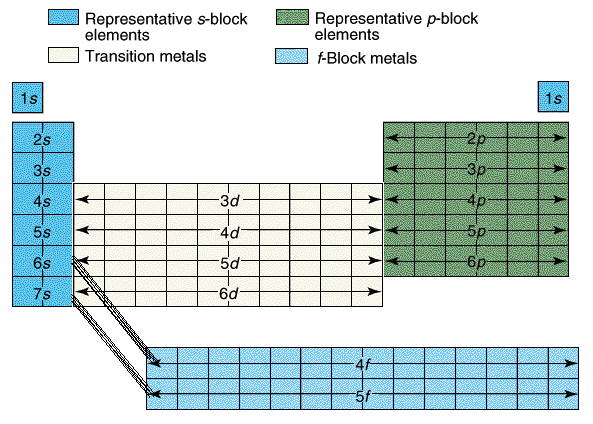
Next students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.
The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
Students practiced identifying the energy level, orbital, and location of elements on the periodic table. For example Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
We finished class with discussing racing into the classroom and getting a seat quickly. These thoughts apply to electrons and how they will fill electron shells. Electrons will sit at the first table first, get their own seat first, and if they must they will share. If they share a seat they have to sit in opposite directions so that they are more stable.
Today in class students learned about electron orbitals. First students learned how many electrons go on the electron shells for a Bohr model.
2 can go on the first ring (corresponding with the 2 elements on the first period of the periodic table). If you look at all the models to the left, they all only have 2 electrons on the first ring near the nucleus. 8 go on the 2nd ring, just like there are 8 elements in the 2nd period. The next ring gets 18.
Next students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.
The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
Students practiced identifying the energy level, orbital, and location of elements on the periodic table. For example Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
We finished class with discussing racing into the classroom and getting a seat quickly. These thoughts apply to electrons and how they will fill electron shells. Electrons will sit at the first table first, get their own seat first, and if they must they will share. If they share a seat they have to sit in opposite directions so that they are more stable.
Thursday, September 9, 2010
Dudes and Periodic Table
Today students started with a BrainPop about the Periodic Table. Tim and Moby went into a little more detail then I did and the quiz was a little more difficult than expected. There is a lot of information on the periodic table and it is set up in all those groups and periods for reasons!
 Today we discussed atom models - what they looked like and what they are called, the dudes who came up with them, and the experiments they did.
Today we discussed atom models - what they looked like and what they are called, the dudes who came up with them, and the experiments they did.
Shrodinger came up with the current electron cloud model, but we draw Bohr's planetary model the most often because it easier to count the electrons.
Electrons are tricky because they move constantly and at high speeds. Heisenberg's Uncertainty Principle states that you cannot know both the speed and location of an electron - you can only know one - becuase measuring either one, changes the other. For more information about the history of atomic models, check out this great link.
We took a short quiz about atoms and elements that students did well on. Everyone got a 7/10 or better... so everyone gets a sticker. :) We finished class with review of the Periodic Table by playing Guess Who. Third period knows enough to play against each other so the rivalries have begun. Check out the photos below.
Homework Tonight - Box A and B on the Gold Sheet
Physical Chemical Labs are due tomorrow.
 Today we discussed atom models - what they looked like and what they are called, the dudes who came up with them, and the experiments they did.
Today we discussed atom models - what they looked like and what they are called, the dudes who came up with them, and the experiments they did.Shrodinger came up with the current electron cloud model, but we draw Bohr's planetary model the most often because it easier to count the electrons.
Electrons are tricky because they move constantly and at high speeds. Heisenberg's Uncertainty Principle states that you cannot know both the speed and location of an electron - you can only know one - becuase measuring either one, changes the other. For more information about the history of atomic models, check out this great link.
We took a short quiz about atoms and elements that students did well on. Everyone got a 7/10 or better... so everyone gets a sticker. :) We finished class with review of the Periodic Table by playing Guess Who. Third period knows enough to play against each other so the rivalries have begun. Check out the photos below.
Homework Tonight - Box A and B on the Gold Sheet
Physical Chemical Labs are due tomorrow.
 |
| Taylor and Taylor go head to head |
 | |
| Karen vs. Brande.... Jasmine vs. Marion... and in the back Shaun vs. James |
 |
| Kaleb vs. Amanda... and Tim vs. Kelsey in the background |
Wednesday, September 8, 2010
Welcome to the Periodic Table
Today students started with a jump in where they identified and counted parts of an atom on drawings. Once they figured out the atomic number (number of protons), they could then tell which atom on the periodic table it was supposed to be.
Students were given a little sample of 5 paint chips and asked to sort them. Most sorted them from lightest to darkest, which was fairly simple because all the paint chips were similar colors. Next students were given a sample with 12 different paint chips and asked to arrange them. Some arranged them in a long line while others put them in groups. Eventually students were asked to put them in three columns of four. Why?
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
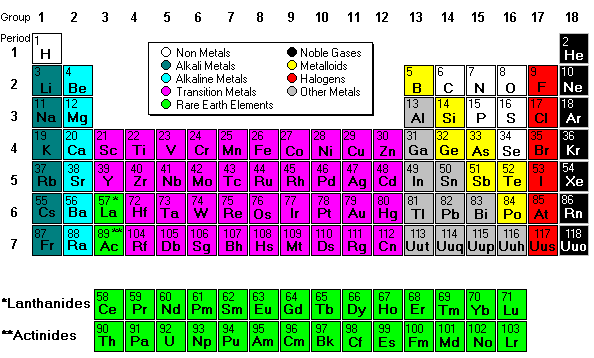
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
In third period, we finished class by playing Guess Who with the Periodic Table in partners. It is a great way to practice naming and identifying the various regions. Some of them were really getting into it which makes the game more interesting. As the students learn the terms they will play against each other instead of asking questions about the element that I have picked.
 Fourth period finished the day with the Physical Chemical Lab from last week (we were interrupted by a fire drill). Many students were able to use the time to finish up the questions on the back as well. Fourth will have opportunities to play Guess Who later this week.
Fourth period finished the day with the Physical Chemical Lab from last week (we were interrupted by a fire drill). Many students were able to use the time to finish up the questions on the back as well. Fourth will have opportunities to play Guess Who later this week.
 We also talked in class about other ways to arrange periodic tables and I found these neat examples. I also like this idea - a periodic table of imaginary elements made up from made-up elements from movies and books - like Superman and Star Trek.
We also talked in class about other ways to arrange periodic tables and I found these neat examples. I also like this idea - a periodic table of imaginary elements made up from made-up elements from movies and books - like Superman and Star Trek.
Students were given a little sample of 5 paint chips and asked to sort them. Most sorted them from lightest to darkest, which was fairly simple because all the paint chips were similar colors. Next students were given a sample with 12 different paint chips and asked to arrange them. Some arranged them in a long line while others put them in groups. Eventually students were asked to put them in three columns of four. Why?
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
In third period, we finished class by playing Guess Who with the Periodic Table in partners. It is a great way to practice naming and identifying the various regions. Some of them were really getting into it which makes the game more interesting. As the students learn the terms they will play against each other instead of asking questions about the element that I have picked.
 Fourth period finished the day with the Physical Chemical Lab from last week (we were interrupted by a fire drill). Many students were able to use the time to finish up the questions on the back as well. Fourth will have opportunities to play Guess Who later this week.
Fourth period finished the day with the Physical Chemical Lab from last week (we were interrupted by a fire drill). Many students were able to use the time to finish up the questions on the back as well. Fourth will have opportunities to play Guess Who later this week. HOMEWORK - Atom Math
Physical/Chemical Lab due Friday
 We also talked in class about other ways to arrange periodic tables and I found these neat examples. I also like this idea - a periodic table of imaginary elements made up from made-up elements from movies and books - like Superman and Star Trek.
We also talked in class about other ways to arrange periodic tables and I found these neat examples. I also like this idea - a periodic table of imaginary elements made up from made-up elements from movies and books - like Superman and Star Trek.
Tuesday, September 7, 2010
Atoms... have you heard of Beanium?
Today students reviewed atoms. Atoms, or elements, are the smallest unit of matter. They retain their identity in chemical reactions and are combined to form compounds and everything in the universe.
 Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
Student reviewed how to determine this information from the periodic table. Then they got a mystery sample of Beanium. They had to count different colors of beans representing subatomic particles and determine which element they had.
Students finished class working on assignments that are due later in the week.
Tonight's homework is to finish an atom reading. Physical/Chemical lab is due Friday.
 Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons). Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
Student reviewed how to determine this information from the periodic table. Then they got a mystery sample of Beanium. They had to count different colors of beans representing subatomic particles and determine which element they had.
Students finished class working on assignments that are due later in the week.
Tonight's homework is to finish an atom reading. Physical/Chemical lab is due Friday.
Thursday, September 2, 2010
Review Day
Today students started with a solid liquid gas matching sheet. Then students reviewed concepts through the use of matching cards. They reviewed Physcial vs. Chemical, States of Matter, Phase Changes, and Elements, Compounds, and Mixtures. Most also did a fifth set of vocabulary terms.
Then we had a little discussion about homework. About half of the students had not turned in their math worksheet the day before. And a lot of the ones turned in were not complete, or not done well. Only one student let me know they were having trouble and came in for zero block for help. Homework needs to be completed well and on time. I wanted to make sure that students understood the math before they had a test on it (the test is tomorrow) and it is hard to do that when work is not turned in.
The rest of class was spent working on the math problems and review sheets in anticipation for tomorrow's test.
Tonight's homework is to make sure that packets are complete - all vocab and problems. Review Sheets need to be finished and Green homework sheets need to be completed. All work is due tomorrow and the test is due tomorrow.
Then we had a little discussion about homework. About half of the students had not turned in their math worksheet the day before. And a lot of the ones turned in were not complete, or not done well. Only one student let me know they were having trouble and came in for zero block for help. Homework needs to be completed well and on time. I wanted to make sure that students understood the math before they had a test on it (the test is tomorrow) and it is hard to do that when work is not turned in.
The rest of class was spent working on the math problems and review sheets in anticipation for tomorrow's test.
Tonight's homework is to make sure that packets are complete - all vocab and problems. Review Sheets need to be finished and Green homework sheets need to be completed. All work is due tomorrow and the test is due tomorrow.
Wednesday, September 1, 2010
Other fourth photos
Physical Chemical Lab
 Today students worked on a lab. Part of the lab was making observations of substances and deciding whether they were elements, compounds, or mixtures.
Today students worked on a lab. Part of the lab was making observations of substances and deciding whether they were elements, compounds, or mixtures.Students also mixed salts and water and measured temperature changes. One of the salts raised the temperature to over 50*C, while another exothermic reaction dropped the temperature to 18*C.
 Other experiments included mixing chemicals and deciding whether the reactions were physical or chemical changes. Most of the reactions were pretty interesting. One of them involved mixing two clear liquids and getting a bright yellow precipitate (a precipitate is a solid!). Students burned small pieces of magnesium ribbon and saw a really bright white light. Stu
Other experiments included mixing chemicals and deciding whether the reactions were physical or chemical changes. Most of the reactions were pretty interesting. One of them involved mixing two clear liquids and getting a bright yellow precipitate (a precipitate is a solid!). Students burned small pieces of magnesium ribbon and saw a really bright white light. StuTonight's Homework is to get a good start on the Unit 2 Review Sheet. TEST FRIDAY
A quick shout out to awesome fourth period who thought it might be cool (or at least a little funny) to wear that lab gear outside for the fire drill.