Students started class with a BrainPop and a worksheet related to Atoms. Scores were very high in all three portions of the competition.
Today in class students learned about electron orbitals. First students learned how many electrons go on the electron shells for a Bohr model.
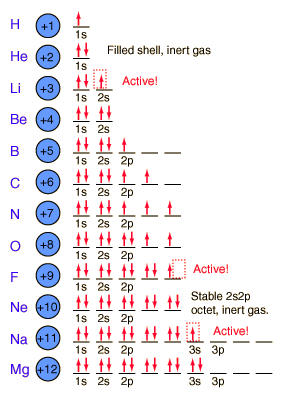
2 can go on the first ring (corresponding with the 2 elements on the first period of the periodic table). If you look at all the models to the left, they all only have 2 electrons on the first ring near the nucleus. 8 go on the 2nd ring, just like there are 8 elements in the 2nd period. The next ring gets 18.
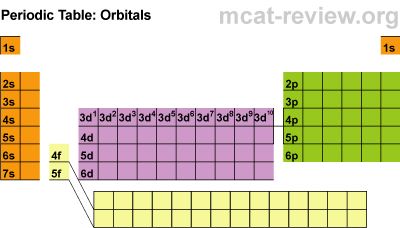
Next students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.
The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
Students practiced identifying the energy level, orbital, and location of elements on the periodic table. For example Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
We finished class with discussing racing into the classroom and getting a seat quickly. These thoughts apply to electrons and how they will fill electron shells. Electrons will sit at the first table first, get their own seat first, and if they must they will share. If they share a seat they have to sit in opposite directions so that they are more stable.

No comments:
Post a Comment
Thanks for your comments :)