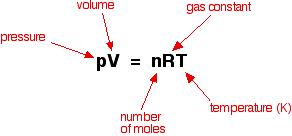
Ideal gases do not actually exist, but we pretend they do and use the Ideal Gas Formula of PV=nRT.
One of these variables will not be given to you and you have to solve for it. This does not seem difficult after stoich, so students dove in, did well, and finished early.
Benchmark III (cumulative) is Friday.
No comments:
Post a Comment
Thanks for your comments :)