Students made a foldable reviewing all the dudes they need to know that influenced the development of the atomic model. Students tried it out without their notes and realized how little they remembered... and then used their notes to record the information. We also watched a BrainPop about the atomic models to help refresh their memories. Now that they have their foldables made, they can use them to study for the unit test and to study for the SOL.
For more information about the evolution of the atomic model, check out this link.
Next we finished up the notes by discussing periodic trends.
Electro- negativity is how badly atoms want electrons. The most electronegative atoms are Fluorine, Chlorine, and Oxygen. Ionization energy is how difficult it is to remove electrons. It is difficult to remove electrons from atoms that are electronegative.
Atomic radius increases as you move down the periodic table because atoms have more mass, but actually decreases from left to right because atoms are holding on to their electrons tighter (because they are more electronegative).
Wednesday, February 23, 2011
Monday, February 21, 2011
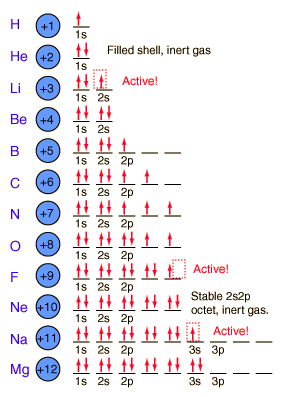
Electronic Configuration
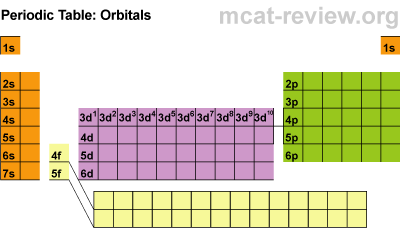 Students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.
Students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
Students learned the pattern of electronic configuration and how to use it. Basically its like giving directions to an element on the PT using set landmarks. It is a bit confusing, but once you get the pattern, its not too bad. We practiced with SPDF and electron configuration with arrows.Students practiced identifying the energy level, orbital, and location of elements on the periodic table. For example Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.
We finished class with discussing racing into the classroom and getting a seat quickly. These thoughts apply to electrons and how they will fill electron shells. Electrons will sit at the first table first, get their own seat first, and if they must they will share. If they share a seat they have to sit in opposite directions so that they are more stable.
Friday, February 18, 2011
Flame Testing
Today students learned about excited electrons through flame testing. Electrons can jump energy levels when they are excited - like when they are caught on fire. They are excited for a bit and then they bounce back down to their normal energy level. The energy the lose when going back is emitted as light.
We watched a short video that explains where and how fireworks are made and what materials are used to make them work. More information about how fireworks work can be found here. For more information and some cool videos check out NOVA's fireworks website .
Different elements and metals emit different spectra of light. Students sampled some metals, tried to identify some unknowns, and then mixed and matched their own to see what color combinations they could make. Some samples worked better than others and most people picked copper as their favorite.
We also started talking about atom models today and the "dudes" who came up with these models and how they did it. More information about this topic will be given on Monday.
Tonight's homework is D and J on the gold sheet.
We watched a short video that explains where and how fireworks are made and what materials are used to make them work. More information about how fireworks work can be found here. For more information and some cool videos check out NOVA's fireworks website .
Different elements and metals emit different spectra of light. Students sampled some metals, tried to identify some unknowns, and then mixed and matched their own to see what color combinations they could make. Some samples worked better than others and most people picked copper as their favorite.
We also started talking about atom models today and the "dudes" who came up with these models and how they did it. More information about this topic will be given on Monday.
Tonight's homework is D and J on the gold sheet.
Thursday, February 17, 2011
Thursday
Today students started with a comprehensive jump in asking questions about all the regions on the periodic table. Students did well though they got frustrated trying to find the elements.
Next we covered notes on putting electrons into orbital shells. The thing to remember is 2, 8, 8, 18. This pattern is also mirrored on the periodic table. It can be more complicated than that, but we got the basics. Pictured are some basics.
Students worked together to go over the Benchmark and to earn team points for the new team. Teams earned points for correct answers and good highlighting. Espada de Dragon, Phineas & Ferb, and Team Awesome are currently in the lead.
We finished class by playing Guess Who. I chose an element and then students took turns asking yes or no questions using the new periodic table terminology they have learned to narrow down the periodic table and guess which element I have chosen.
Homework for tonight is the Gold Homework sheet boxes ABC.
Tomorrow we are doing the flame lab - bring appropriate clothing and please wear glasses instead of contacts.
Next we covered notes on putting electrons into orbital shells. The thing to remember is 2, 8, 8, 18. This pattern is also mirrored on the periodic table. It can be more complicated than that, but we got the basics. Pictured are some basics.
Students worked together to go over the Benchmark and to earn team points for the new team. Teams earned points for correct answers and good highlighting. Espada de Dragon, Phineas & Ferb, and Team Awesome are currently in the lead.
We finished class by playing Guess Who. I chose an element and then students took turns asking yes or no questions using the new periodic table terminology they have learned to narrow down the periodic table and guess which element I have chosen.
Homework for tonight is the Gold Homework sheet boxes ABC.
Tomorrow we are doing the flame lab - bring appropriate clothing and please wear glasses instead of contacts.
Wednesday, February 16, 2011
Welcome to Atoms and the Periodic Table
We have taken and passed the Unit 2 Test (Friday) and taken and passed the first Benchmark (Tuesday). We are now in new seats and learning about atoms and the periodic table.
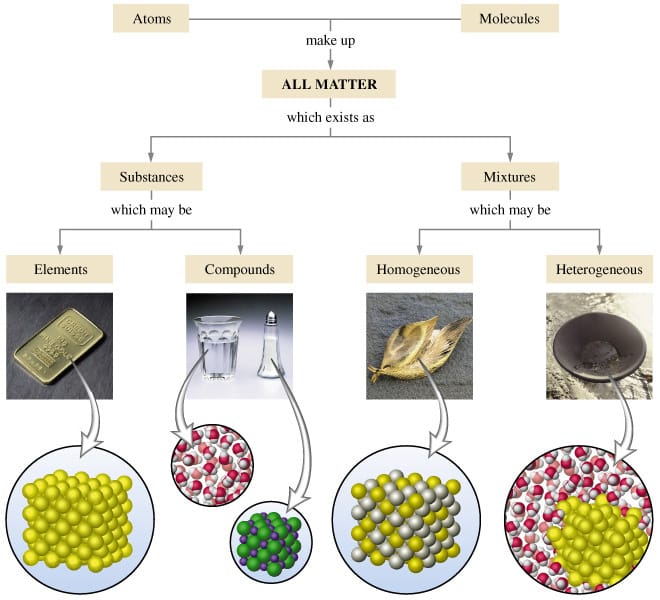
Atoms, or elements, are the smallest unit of matter. They retain their identity in chemical reactions and are combined to form compounds and everything in the universe.
 Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
Students got a sample of beans and counted the number of protons, neutrons, and electrons to see which atom it matched with on the periodic table.
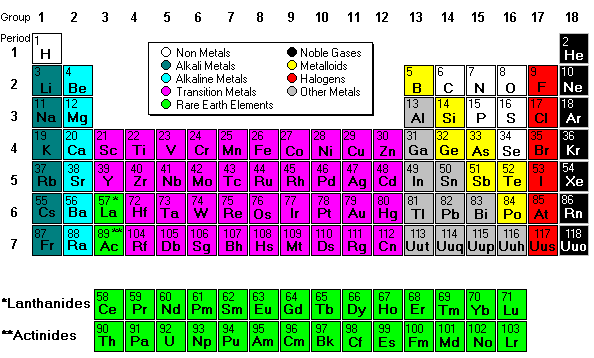
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
First and fourth took a quick pop quiz about determining atom information from a periodic table square. We finished class by playing a quick game of Point where students competed against others at their table to quickly point at the regions they have learned. Tomorrow we will take identification to the next level and play Guess Who.
Atoms, or elements, are the smallest unit of matter. They retain their identity in chemical reactions and are combined to form compounds and everything in the universe.
 Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons). Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
Students got a sample of beans and counted the number of protons, neutrons, and electrons to see which atom it matched with on the periodic table.
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
First and fourth took a quick pop quiz about determining atom information from a periodic table square. We finished class by playing a quick game of Point where students competed against others at their table to quickly point at the regions they have learned. Tomorrow we will take identification to the next level and play Guess Who.
Homework tonight - Atom Math
Lab Friday - NO contacts
Unit 3 test next Thursday the 24th (?)
Monday, February 14, 2011
Wednesday, February 9, 2011
Heat Heat
Today students learned about endo and exothermic reactions.
To test this out, students in groups were given a whack-a-pack and asked to make observations. The pack starts off at room temperature and when you hit it, the reaction occurs. This is a chemical reaction for a few reasons - one you can hear it fizzing. Two it blows up so a gas is being formed (1 of the 4 ways you know a chemical reaction has occurred). And Three there is a temperature change (another of the four ways). The pack gets really cold which means it is releasing heat and this is an exothermic reaction.
Watch this little video to see how it works. These are available at Dollar Tree if you are interested.I will try to post some student photos soon.
After this lab demo, students answered questions and then worked on math practice.
Tonight's homework is the Unit 2 Review Sheet (it's tan). The Unit 2 test is Friday. The first Benchmark will be on Tuesday.
Endothermic reactions absorb heat and get warmer (End Up).
Exothermic reactions lose or release heat and get colder (Exit down).
To test this out, students in groups were given a whack-a-pack and asked to make observations. The pack starts off at room temperature and when you hit it, the reaction occurs. This is a chemical reaction for a few reasons - one you can hear it fizzing. Two it blows up so a gas is being formed (1 of the 4 ways you know a chemical reaction has occurred). And Three there is a temperature change (another of the four ways). The pack gets really cold which means it is releasing heat and this is an exothermic reaction.
Watch this little video to see how it works. These are available at Dollar Tree if you are interested.I will try to post some student photos soon.
After this lab demo, students answered questions and then worked on math practice.
Tonight's homework is the Unit 2 Review Sheet (it's tan). The Unit 2 test is Friday. The first Benchmark will be on Tuesday.
Tuesday, February 8, 2011
Dilutions
Today we learned about dilutions. The formula is M1V1=M2V2. These questions will mention molarity twice and volume twice and will not mention moles. Three numbers are given and the fourth needs to be solved for by isolating it through division.
When diluting substances the molarity (concentration) decreases or becomes more dilute because more water is added to the original solution. The number of moles of solute stays the same, but the molarity changes because the volume increases.
Physical Chemical labs were due today.
Homework tonight is to finish the Green Sheet and work on the Vocabulary.
Unit 2 Test Friday
Benchmark (Units 1 and 2 cumulative) on Tuesday
When diluting substances the molarity (concentration) decreases or becomes more dilute because more water is added to the original solution. The number of moles of solute stays the same, but the molarity changes because the volume increases.
Physical Chemical labs were due today.
Homework tonight is to finish the Green Sheet and work on the Vocabulary.
Unit 2 Test Friday
Benchmark (Units 1 and 2 cumulative) on Tuesday
Monday, February 7, 2011
Solutions, Suspensions, and Colloids... Oh My!
Today we started with a math jump in reviewing specific heat and heat conversions we learned on Friday. We got through it, but we will be practicing again tomorrow.
Today in notes we started with discussing solutions, suspensions and colloids. Solutions are homogeneous well-mixed mixtures that cannot be separated easily (a change in phase) - like kool-aid or saltwater. Suspensions will settle and separate over time because of gravity or because of differences in density - like oil and water or orange juice with pulp. Suspensions are heterogeneous. Colloids are weird. Colloids should settle and separate over time but because the particles are super-tiny just running into each other (Brownian motion) keeps them in suspension. Colloids can also represent two different phases so if it seems weird like you cannot classify it as just one phase - like fog, jello, whipped cream - it's a colloid.
Next we discussed colligative properties. If you add solute to a solution, like salt to water, it changes the properties of the solution, particularly the boiling point or freezing point. We put salt on the roads to lower the freezing point of water so ice does not form on the roads.
Electrolytes can conduct electricity because the solute breaks up into ions and the ions can carry the electric current. Pure water does not conduct electricity - but water with solutes in it can. We did an in-class demo similar to this one to test some solutions. Salt water does conduct electricity, but sugar water does not because of the carbon. Gatorade conducts electricity but barely because of the high sugar amount in the drink.
Finally we talked about Molarity. Molarity is moles/Liters and is a quantitative way to measure concentration. Molarity descirbes with numbers if a solution is dilute or concentrated. It is a pretty easy formula so students zoomed through it. Molarity changes with the amount of solute OR the amount of solvent (liquid) so we will be discussing dilutions tomorrow.
In some classes we got to the team challenge on solutions, suspensions, and colloids, and in some classes we did not, so we will finish that up tomorrow.
Homework tonight = green sheet KLM
Test Friday
Benchmark Tuesday covering units 1 and 2.
Friday, February 4, 2011
Math and more math
Today we spent the first half of class going over the math homework (heat calculations) from the previous evening. Students realized that it wasn't as hard as they thought it was and now feel more confident with reading directions and dissecting math problems. Groups did three challenge questions on the topic to see how many points they can add to their team scores.
For notes today we discussed vapor pressure and boiling point. The boiling point of a liquid is when the vapor pressure equals the external pressure. When the pressures are equal it is easier for liquids to boil and vaporize into gases and steam away. We discussed definitions and answered questions about vapor pressure graphs. This graph is similar - just know that Standard Temperature is 760 torr and 101.3kPa.
To finish class, we finished the chemical and physical lab. Students were really good about getting their stuff together quickly and finishing their last stations.
Tonight's homework is a review sheet for Unit 1 in the form of released SOL questions. This is familiar information, so students should do well on it.
For notes today we discussed vapor pressure and boiling point. The boiling point of a liquid is when the vapor pressure equals the external pressure. When the pressures are equal it is easier for liquids to boil and vaporize into gases and steam away. We discussed definitions and answered questions about vapor pressure graphs. This graph is similar - just know that Standard Temperature is 760 torr and 101.3kPa.
To finish class, we finished the chemical and physical lab. Students were really good about getting their stuff together quickly and finishing their last stations.
Tonight's homework is a review sheet for Unit 1 in the form of released SOL questions. This is familiar information, so students should do well on it.
Thursday, February 3, 2011
Physical Chemical Lab
Today students started with a Jump In practicing all the stuff we talked about yesterday - substances and mixtures. One paper from each table was turned in for points towards each team. These tallies will be updated tomorow.
Students took a Thursday Quiz on the material that has been covered and so far they look pretty good.
The rest of class was spent on the Physical and Chemical Lab. Students mixed chemicals, added water, heated substances, and burned chemicals. Students made observations and tried to decide whether the changes were chemical or physical.
We did not finish the lab today and will finish the last station tomorrow.
Homework tonight is a Heat Calculation Worksheet.
Students took a Thursday Quiz on the material that has been covered and so far they look pretty good.
The rest of class was spent on the Physical and Chemical Lab. Students mixed chemicals, added water, heated substances, and burned chemicals. Students made observations and tried to decide whether the changes were chemical or physical.
We did not finish the lab today and will finish the last station tomorrow.
Homework tonight is a Heat Calculation Worksheet.
Wednesday, February 2, 2011
Matter
Today we discussed matter. Everything in the universe is made up of matter. Matter can be classified as substances of mixtures. A substance is pure and is either made up of elements, the simplest form of matter found on the periodic table, or compounds - two or more atoms chemically combined that can be represented with a formula (ie. H2O).
A mixture is two or more things (substances or anything really) in the same place at the same time. Salt water is a tricky one because we know we can write a formula for salt (NaCl) and water (H2O), but it is a mixture because when mixed in solution, these compounds do not combine. Mixtures can be heterogeneous of homogeneous based on how the solute is spread through the mixture. Heterogeneous mixtures are not mixed evenly and each sample could be different - like salad, chocolate chip cookie dough ice cream, air, and soil. Homogeneous mixtures are the same throughout like creamy peanutbutter, vanilla ice cream, pure air, sugar, and kool-aid.

One important thing we discussed today was the Law of Conservation of Matter proposed by Lavoisier. Basically matter cannot be created or destroyed... it is conserved or recycled or moved somewhere else, but it cannot magically appear or disappear!
After discussing these ways to classify matter, teams worked on sorting matter. Instead of doing this all at once we did this in four rounds, first asking them to classify substances versus mixtures, then classifying the the substances as elements or compounds, then the mixtures as homogeneous or heterogeneous and finally another mixture sort just to make sure they got it.
We finished class by discussing specific heat and heat capacity. I set up a demonstration specific heat problem and students practiced their own.
A mixture is two or more things (substances or anything really) in the same place at the same time. Salt water is a tricky one because we know we can write a formula for salt (NaCl) and water (H2O), but it is a mixture because when mixed in solution, these compounds do not combine. Mixtures can be heterogeneous of homogeneous based on how the solute is spread through the mixture. Heterogeneous mixtures are not mixed evenly and each sample could be different - like salad, chocolate chip cookie dough ice cream, air, and soil. Homogeneous mixtures are the same throughout like creamy peanutbutter, vanilla ice cream, pure air, sugar, and kool-aid.
One important thing we discussed today was the Law of Conservation of Matter proposed by Lavoisier. Basically matter cannot be created or destroyed... it is conserved or recycled or moved somewhere else, but it cannot magically appear or disappear!
After discussing these ways to classify matter, teams worked on sorting matter. Instead of doing this all at once we did this in four rounds, first asking them to classify substances versus mixtures, then classifying the the substances as elements or compounds, then the mixtures as homogeneous or heterogeneous and finally another mixture sort just to make sure they got it.
We finished class by discussing specific heat and heat capacity. I set up a demonstration specific heat problem and students practiced their own.
Tonight's homework is box D and E on the Green Sheet
Physical-Chemical Lab tomorrow - Write-up due Tuesday
Quiz tomorrow because its Thursday
Test sometime next week.
Tuesday, February 1, 2011
Solutions
Students began class with a jump in about the differences between solids, liquids, and gases and checked their answers. We went over the density homework and answered any questions about how to set up the equation and determine the units.
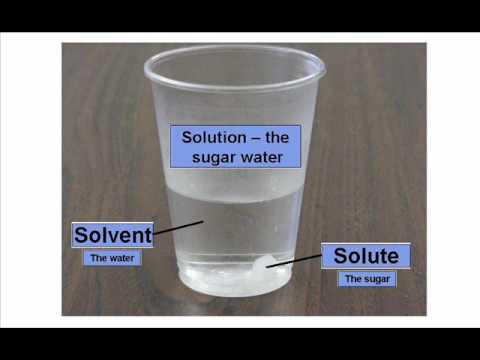
Today in class we discussed solutions. Solutions are homo- geneous mixtures comprised of solutes dispersed in a solvent. Water is the universal solvent, but not the only solvent. For example, a marshmallow is a solid (sugar) solute dispersed in a gaseous solvent (air).
Solubility is how well something dissolves. Some things are very soluble, and some are insoluble (do not dissolve).
Solutions are said to be saturated if they are holding all the solute that they can. When the solute starts to build up on the bottom, you know a solution is definitely saturated (like the dark blue solution on the right). Solutions are unsaturated if they can dissolve more solute (like the two light blue solutions on the left).
Solutions can be super-saturated if they are heated because they can hold more solute than normal. Even if you cool these solutions back down, they will still hold this additional solute in solution. Sweet Tea and all candies are made by first making super-saturated solutions and then cooling them.
For an excellent website about all of these topics and others regarding solutions that have and will be covered in this unit - check out this useful website.
Tonight's homework is entitled "JI Solutions" and was given yesterday just in case it snowed/sleeted.
There will be a lab on THURSDAY. Please make sure students are dressed appropriately and make every effort to be in class.