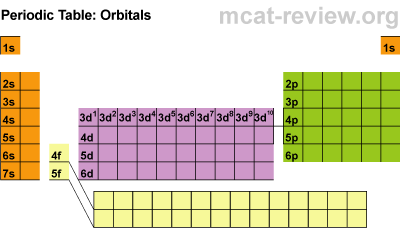
 Students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.
Students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
Students learned the pattern of electronic configuration and how to use it. Basically its like giving directions to an element on the PT using set landmarks. It is a bit confusing, but once you get the pattern, its not too bad. We practiced with SPDF and electron configuration with arrows.Students practiced identifying the energy level, orbital, and location of elements on the periodic table. For example Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.
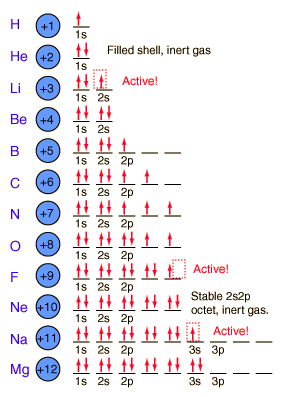
We finished class with discussing racing into the classroom and getting a seat quickly. These thoughts apply to electrons and how they will fill electron shells. Electrons will sit at the first table first, get their own seat first, and if they must they will share. If they share a seat they have to sit in opposite directions so that they are more stable.
No comments:
Post a Comment
Thanks for your comments :)