 Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different.
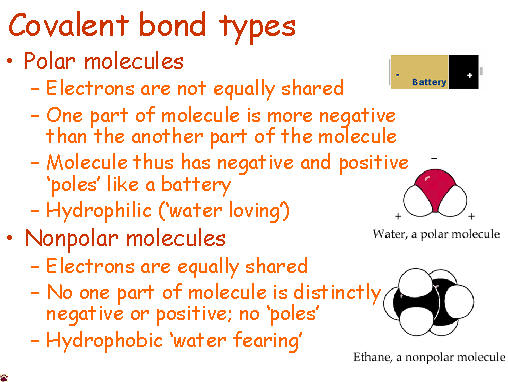
Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different. If a molecules is nonpolar covalent, it is sharing its electrons equally. The best example of this is in diatomic molecules. Diatomic molecules are two of the same atom bonded together - so they would have exactly the same pull. Symmetrical molecules are also nonpolar.
Polar covalent bonds occur when electrons are not equally shared. One atom, usually more electronegative, has a stronger pull on the electrons and shares them unequally. The other atom that is less electronegative has a smaller hold on the electrons and is thus can be slightly positive.
One way to remember this is... "Polar Bears do not share... equally."

No comments:
Post a Comment
Thanks for your comments :)