Class began as normal with students retrieving their homework and starting on two conversion problems. Then the room began to shake. It took me a few moments to register what was happening, then once I realized it was an earthquake I told the students to get under their desks. From what I have read online the shaking lasted about 45 seconds, but anyone who was there knows it felt much longer.
The room was still unsteady when the fire alarms started to go off. I had my students wait a few moments so I could relay information. I had them take their backpacks, cautioned them to be careful, quick, and calm. My students were excellent. It took me a few moments to grab my phone, keys, and name sign and I was one of the last down the stairs. Students were exiting very quickly and taking it well. One girl's backpack fell open and three boys stopped grabbed all her things and kept walking.
My students met me at the bottom of the hill, our prearranged meeting location and sat quietly. I answered any questions I could about earthquakes and what was happening. While we waited in the sunshine we could hear and feel aftershocks. I am very proud of my students for staying calm and sharing phones so that others could try to get in touch with their loved ones.
After the buses had arrived students walked calmly to the bus loop and got on the buses to go home. Students that drove to school and had their keys were allowed to get in their own vehicles and drive home. On the whole, I am amazed how calm everyone was during a situation that does not frequently occur in Virginia.
Currently all buildings are locked and being patrolled by the local authorities so any belongings left by students are safe. When students are allowed to re-enter the building that information will be passed along. Please enjoy your time off until school resumes the Tuesday after Labor Day.
Wednesday, August 24, 2011
Monday, August 22, 2011
To begin class we went over the weekend's homework and discussed lab techniques.
Next, a few volunteers threw squishy whales at a bullseye. We then discussed who was the closest to the middle and who was the most consistent. This related to accuracy (how close a measurement is to the real measurement) and precision (how close a set of points are to each other) which is what we discussed first. We did some examples as a class and then tied accuracy and precision to percent error.
We practiced calculating percent error by measuring each other's ears and comparing the measurements to the correct value as measured by MsJ.
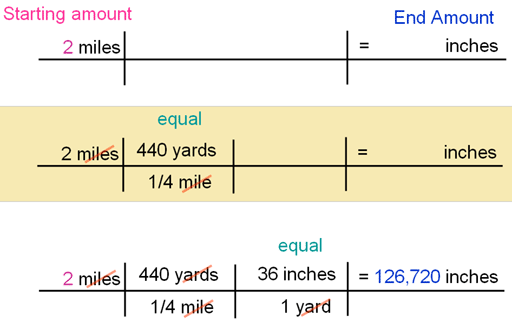
We finished class with some dimensional analysis. Dimensional analysis (or train tracks) is used to convert from one unit to another. Right now we are just learning the process, but these conversions are very important in chemistry and even have their own special name in that regard - stoichiometry.
The important thing to remember in conversions is to match the units diagonally (and usually down) so they cancel and you are able to convert to the new unit you are trying to get to. Some conversions are going to take more than one step. For right now we are doing a mix of standard conversions and completely made up ones.
Anyone who needs help with this needs to let me know! This website has a pretty good explanation.
Anyone who needs help with this needs to let me know! This website has a pretty good explanation.
Homework for this evening is to measure some items around the house using no rulers! We will be doing our first chemistry lab on Wednesday.
Friday, August 19, 2011
Gummy Bear Conclusions
Today students got to see what happened when gummy bears are soaked in water for 24 hours. They get huge! Students could see that the gummy bears were larger, but really had a good idea of the size change when they compared their big bear to a control, a normal size gummy bear.
Morgan's bear did not get bigger because he used the sink with hot water. His gummy bear dissolved!
Some students had difficulties measuring the gigantic bears because now they were more fragile and quite a few lost feet and some bears were just blobs. Students worked on their measurements and writing good conclusions.The gummy bears absorbed water and as a result gained in volume and became less dense because the particles moved further apart.
Some students had difficulties measuring the gigantic bears because now they were more fragile and quite a few lost feet and some bears were just blobs. Students worked on their measurements and writing good conclusions.The gummy bears absorbed water and as a result gained in volume and became less dense because the particles moved further apart.
Thursday, August 18, 2011
Lab Equipment and Gummy Bears
The winners of the lab equipment challenge were Moe, Seth, and Adam. They were given a wordbank (that was honestly incomplete) and a room full of numbered lab equipment and challenged to identify it all.
Today students started a lab involving the scientific method and measuring gummy bears. The students got their bears and measured them and then covered them in water to wait. While waiting students identified lab equipment and took their Thursday Quiz.
 While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.
While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.
Everyone complains they can't eat the bears at the beginning, but complain they don't want to eat them at the end. The cool thing is - there is one more bear in the wings sitting for 24 hours... what is going to happen to it?
Homework was a handout on lab equipment and there is a safety test tomorrow.
Today students started a lab involving the scientific method and measuring gummy bears. The students got their bears and measured them and then covered them in water to wait. While waiting students identified lab equipment and took their Thursday Quiz.
 While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.
While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.Everyone complains they can't eat the bears at the beginning, but complain they don't want to eat them at the end. The cool thing is - there is one more bear in the wings sitting for 24 hours... what is going to happen to it?
Homework was a handout on lab equipment and there is a safety test tomorrow.
Wednesday, August 17, 2011
First Week - Density and Safety
Monday was a get-to-know-you sort of day. Students started with an informal survey and drew a picture of themselves that I will use to help learn their names (I am terrible at names). I familiarized the students with classroom procedures, discussed homework and classwork, and let them know how class was going to run.
We have made sure that everyone had a good handle on density in regards to definition, math and formulas, and what it actually means. Density is how close together the particles are in a substance. If they are close together the substance is more dense. If the particles are far apart, the substance is less dense. I do not float in Lake Anna, but I do float in the ocean - therefore I am more dense than Lake Anna and less dense than the ocean.
Students made predictions for a density column including corn syrup, water, mouthwash, baby oil, and vegetable oil. Things that are more dense-sink, things that are less dense-rise to the top, things with similar densities-mix. Then I poured the liquids in a randomly asked for order and students looked at how the layers formed because of the differences in density. They also fielded oral questions as the demonstration was performed. Here is a photo of a similar demo.
Today we are going to cover lab equipment and do a fun gummy bear lab. Here is a good handout to review them. Students will try on their own to id the equipment before I tell them what everything is.
Last night's homework was box A&B on the blue sheet. Tonight it is box C&D. Tomorrow is Thursday so there is a syllabus/density quiz and the safety test will be on Friday.
Tuesday, August 9, 2011
Welcome Back! Fall 2011
Greetings students, parents, and guardians.
Welcome to a new school year with Ms Jancaitis! This blog has been set up to connect students, parents, and guardians with the chemistry class.
At Open House or in class, each student will receive a course syllabus, safety rules, and a breakage sheet. The safety rules and breakage sheet needs to be read and signed by both the student and parent guardian.
Welcome to a new school year with Ms Jancaitis! This blog has been set up to connect students, parents, and guardians with the chemistry class.
At Open House or in class, each student will receive a course syllabus, safety rules, and a breakage sheet. The safety rules and breakage sheet needs to be read and signed by both the student and parent guardian.
- The course syllabus outlines what the course will be like, what topics will be covered, and course expectations. It also contains contact information. There will be a quiz on this syllabus on Thursday.
- The safety rules are rules designed to keep the classroom safe and orderly to maximize learning and prevent accidents and injuries. These rules need to be studied because there will be a safety test on Friday and infractions of these rules can lead to disciplinary action as well as low assignment grades.
- A breakage sheet is a contract holding students accountable for the items that are broken if the student is acting a manner that is unsafe for themselves or those around them.