To begin class we went over the weekend's homework and discussed lab techniques.
Next, a few volunteers threw squishy whales at a bullseye. We then discussed who was the closest to the middle and who was the most consistent. This related to accuracy (how close a measurement is to the real measurement) and precision (how close a set of points are to each other) which is what we discussed first. We did some examples as a class and then tied accuracy and precision to percent error.
We practiced calculating percent error by measuring each other's ears and comparing the measurements to the correct value as measured by MsJ.
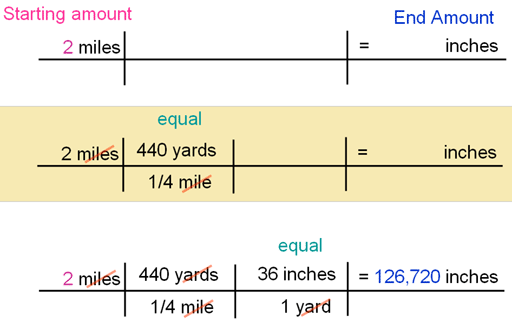
We finished class with some dimensional analysis. Dimensional analysis (or train tracks) is used to convert from one unit to another. Right now we are just learning the process, but these conversions are very important in chemistry and even have their own special name in that regard - stoichiometry.
The important thing to remember in conversions is to match the units diagonally (and usually down) so they cancel and you are able to convert to the new unit you are trying to get to. Some conversions are going to take more than one step. For right now we are doing a mix of standard conversions and completely made up ones.
Anyone who needs help with this needs to let me know! This website has a pretty good explanation.
Anyone who needs help with this needs to let me know! This website has a pretty good explanation.
Homework for this evening is to measure some items around the house using no rulers! We will be doing our first chemistry lab on Wednesday.


No comments:
Post a Comment
Thanks for your comments :)