Today, we started talking about moles. Moles are used to count atoms. There are 22,000,000,000,000,000,000 quintillion atoms in a grain of sand and even counting grains of sand is a pain. Because atoms are so tiny, we use the mole to estimate.
There are 6.02 x 10 ^23 molecules in one mole. That's a whole lot. This is our new favorite number because it needs to be memorized. We practiced converting from moles to molecules.
Next we discussed molar mass. Molar mass = 1 mole and it also equals atomic mass from the periodic table. To find the molar mass of carbon dioxide you find the mass of carbon and two oxygens and add them together. Finding molar mass is not difficult unless the molecule has tricky subscripts (which we have been practicing).
Monday, December 5, 2011
Friday, December 2, 2011
Le Chatlier
Students learned about reaction rates and how to increase them. They also learned about reversible reactions and how Le Chatlier's principle influences shifts of equilibrium in reversible reactions.
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
 Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
 Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Wednesday, November 30, 2011
Reaction Rate Basics
Reaction Rates are affected by a few things. Without telling them the point, the students had a quick demo where they had to dissolve sugar cubes the fastest.
The things that speed up reactions are:
- Temperature - warmer is faster
- Surface Area - small pieces have more surface area
- Concentration - the more water, the faster sugar will dissolve
- Catalyst - lowers the activation energy and speeds up the reaction
- Agitation - shaking or stirring increases the frequency of collisions.
Monday, November 28, 2011
Reaction Types
We started by talking about the simple definition of the terms, what the probably products and reactants are and went over a basic formula for the reaction types the students need to be familiar with.
Reaction Types include:
Homework is to finish the Benchmark Review Sheet and J and I on the orange homework sheet
Reaction Types include:
- synthesis
- decomposition
- singe replacement
- double replacement
- combustion
- endothermic
- exothermic
- oxidation-reduction
- neutralization
Homework is to finish the Benchmark Review Sheet and J and I on the orange homework sheet
Wednesday, November 23, 2011
Balancing Equations
Students are learning to balance equations. Today they learned that reactants are what you start with and are on the left side of the equation. Products are on the right side of the arrow and are what is made by process of a chemical change.
Because of the Law of Conservation of Mass, the number of atoms have to be equal on both sides. To balance an equation, the coefficients are changed. Coefficients are the big numbers in front that tell you how many molecules there are. The subscripts (the little lower numbers) are not allowed to be changed because those are there to make neutrally bonded molecules (what we learned in the last unit.
By changing the coefficients and counting the number of atoms on both sides of the arrow, balancing can be achieved.
Because of the Law of Conservation of Mass, the number of atoms have to be equal on both sides. To balance an equation, the coefficients are changed. Coefficients are the big numbers in front that tell you how many molecules there are. The subscripts (the little lower numbers) are not allowed to be changed because those are there to make neutrally bonded molecules (what we learned in the last unit.
By changing the coefficients and counting the number of atoms on both sides of the arrow, balancing can be achieved.
Saturday, November 5, 2011
Polar vs NonPolar
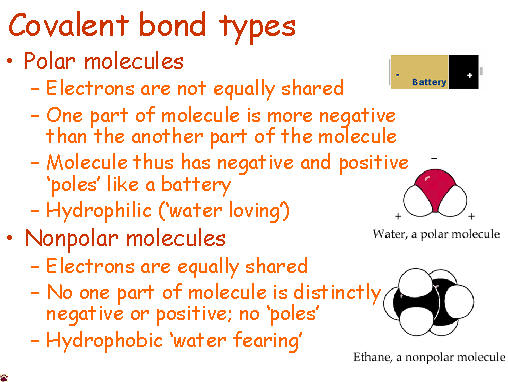 Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different.
Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different. If a molecules is nonpolar covalent, it is sharing its electrons equally. The best example of this is in diatomic molecules. Diatomic molecules are two of the same atom bonded together - so they would have exactly the same pull. Symmetrical molecules are also nonpolar.
Polar covalent bonds occur when electrons are not equally shared. One atom, usually more electronegative, has a stronger pull on the electrons and shares them unequally. The other atom that is less electronegative has a smaller hold on the electrons and is thus can be slightly positive.
One way to remember this is... "Polar Bears do not share... equally."
Sunday, October 30, 2011
Friday, October 28, 2011
Covalent Bonding
We are practicing ionic bonding and naming and learning how to bond and name things that are covalent.
If it is a - and -, the bond is covalent. The electrons are shared in the bond. To get the formula, you have to draw the Lewis Dot structures for the elements and connect the dots that don't have friends. You write the formula based on your drawing. To name it, use prefixes to indicate the number of atoms in the formula and the second one ends in -ide. For these it doesn't matter which element comes first.
If it is a - and -, the bond is covalent. The electrons are shared in the bond. To get the formula, you have to draw the Lewis Dot structures for the elements and connect the dots that don't have friends. You write the formula based on your drawing. To name it, use prefixes to indicate the number of atoms in the formula and the second one ends in -ide. For these it doesn't matter which element comes first.
Tuesday, October 25, 2011
Ionic Bonding
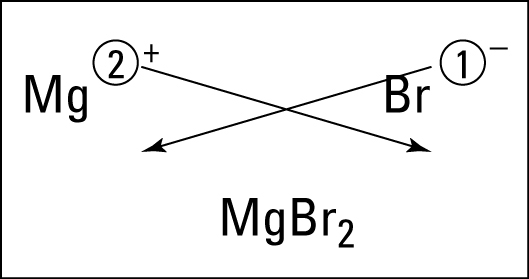
Students learned about ionic bonding. Ionic bonding happens between metals & nonmetals (positives & negatives). The electrons are given and taken in this ionic bond. To get the formula, you criss cross the charges. To name it, you say the name of the metal, then the name of the nonmetal with an -ide ending. If it is a metal from DForP block, then you use a roman numeral to indicate the charge of the metal.
 After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods.
After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods.
 Now that we understand ionic bonding, students should find this cartoon amusing.
Now that we understand ionic bonding, students should find this cartoon amusing.
Ionic Bonds for Dummies
Here is a cool interactive where you can build models to simulate ionic bonding.
 After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods.
After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods. Now that we understand ionic bonding, students should find this cartoon amusing.
Now that we understand ionic bonding, students should find this cartoon amusing. Ionic Bonds for Dummies
Here is a cool interactive where you can build models to simulate ionic bonding.
Sunday, October 23, 2011
Valence Electrons and the charge of ions
Yesterday students learned about valence electrons. Valence electrons are the outermost electrons and are the electrons that are used for bonding and participate in reactions. Valence electrons are only found in the S and P blocks. The max number of valence electrons is 8. Students practiced counting valence electrons and drawing Lewis Dot Structures.
Students also practiced identifying which noble gas an element wanted to be like. All elements want to be like two noble gases - it is just a matter of figuring out which is closer. Elements want to be like noble gases because they have full outer electron shells, or full valences. This makes them stable and non reactive which is why noble gases are sometimes called the inert gases.
Today students learned how to use valence electrons and dot structures to determine the charge of an atom. Atoms either want to gain electrons or lose electrons to become like those noble gases they envy.
Students also practiced identifying which noble gas an element wanted to be like. All elements want to be like two noble gases - it is just a matter of figuring out which is closer. Elements want to be like noble gases because they have full outer electron shells, or full valences. This makes them stable and non reactive which is why noble gases are sometimes called the inert gases.
Today students learned how to use valence electrons and dot structures to determine the charge of an atom. Atoms either want to gain electrons or lose electrons to become like those noble gases they envy.
Students also learned how to identify the charges of metals with more than one oxidation state using Roman numerals. Metals in the D, F, and lower P get Roman numerals - basically all metals but the S block, Aluminum and Boron get roman numerals. The roman numeral tells you the charge. We have to use this system because those odd metals can actually be found in more than one form - some with 2 possible charges - some with more than four!
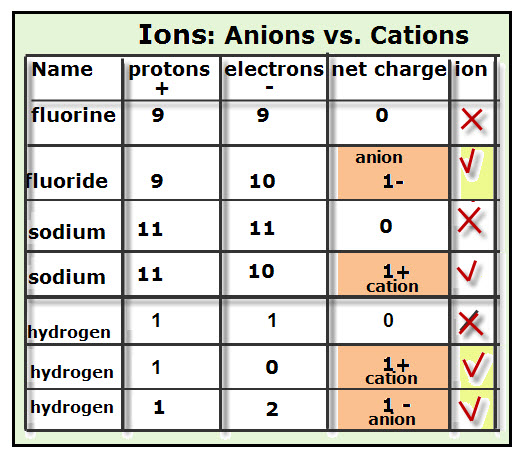
- Ions are atoms or molecules that have a net charge, either positive or negative. There are two kinds of ions:
- Anions are negatively charged ions because they have negative net charges. This means that there is a greater number of electrons (-) than protons (+). For example, the anion, fluoride (F 1-), has a one negative charge because it has a total of nine protons and ten electrons. Thus, the net charge for fluoride is 1 negative.
- Cations are positively charged ions because they have positive net charges. This is due to these ions having more protons (positive charges) than electrons (negative charges). For example, calcium (Ca 2+) is a cation ion with 20 protons and 18 electrons. The net charge for Calcium is 2 positive. (from here)
Wednesday, October 19, 2011
Atom Models and Periodic Trands
For more information about the evolution of the atomic model, check out this link.
Periodic Trends.
Electro- negativity is how badly atoms want electrons. The most electronegative atoms are Fluorine, Chlorine, and Oxygen. Ionization energy is how difficult it is to remove electrons. It is difficult to remove electrons from atoms that are electronegative.
Atomic radius increases as you move down the periodic table because atoms have more mass, but actually decreases from left to right because atoms are holding on to their electrons tighter (because they are more electronegative).
Monday, October 17, 2011
Electronic Configuration & Battleship
Electronic Configuration
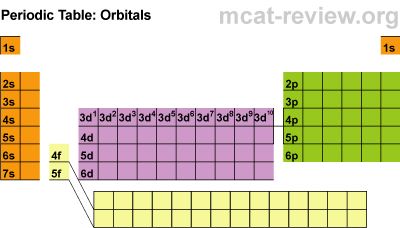 Students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.
Students learned about the orbitals SPDF and what regions of the periodic table those orbitals correspond with. The letters really have to do with an intense mathematical equation used to calculate the probability of finding an electron in the electron cloud.The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
Students learned the pattern of electronic configuration and how to use it. Basically its like giving directions to an element on the PT using set landmarks. It is a bit confusing, but once you get the pattern, its not too bad. We practiced with SPDF and electron configuration with arrows.Students practiced identifying the energy level, orbital, and location of elements on the periodic table. For example Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.
Friday, October 7, 2011
Atoms and the Periodic Table
Atoms, or elements, are the smallest unit of matter. They retain their identity in chemical reactions and are combined to form compounds and everything in the universe.
 Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
Students got a sample of beans and counted the number of protons, neutrons, and electrons to see which atom it matched with on the periodic table.
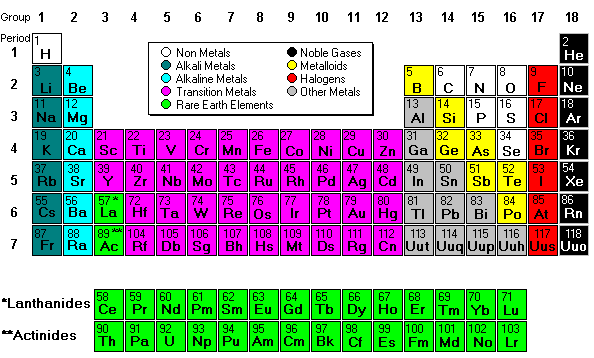
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
We finished class by playing a quick game Guess Who.
 Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons).
Atoms have some basic parts. Protons and Neutrons are found in the nucleus and make up the atomic mass. To find the number of neutrons, you subtract the atomic number (number of protons) from the atomic mass number (protons plus neutrons). Electrons are so tiny that they do not influence the atomic mass. They are found orbiting the nucleus in shells or orbitals. Atoms are neutral so the number of protons equals the number of electrons.
Students got a sample of beans and counted the number of protons, neutrons, and electrons to see which atom it matched with on the periodic table.
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram. This website gives a lot of helpful information.
We finished class by playing a quick game Guess Who.
Saturday, October 1, 2011
Endo and Exo
Today students learned about endo and exothermic reactions.
Watch this little video to see how it works. These are available at Dollar Tree at Valentine's Day if you are interested.
Endothermic reactions absorb heat and get warmer (End Up).
Exothermic reactions lose or release heat and get colder (Exit down).
To test this out, look at a whack-a-pack and make observations. The pack starts off at room temperature and when you hit it, the reaction occurs. This is a chemical reaction for a few reasons - one you can hear it fizzing. Two it blows up so a gas is being formed (1 of the 4 ways you know a chemical reaction has occurred). And Three there is a temperature change (another of the four ways). The pack gets really cold which means it is releasing heat and this is an exothermic reaction.
Watch this little video to see how it works. These are available at Dollar Tree at Valentine's Day if you are interested.
Thursday, September 29, 2011
Properties of Solutions
Next we discussed colligative properties. If you add solute to a solution, like salt to water, it changes the properties of the solution, particularly the boiling point or freezing point. We put salt on the roads to lower the freezing point of water so ice does not form on the roads.
Electrolytes can conduct electricity because the solute breaks up into ions and the ions can carry the electric current. Pure water does not conduct electricity - but water with solutes in it can. We did an in-class demo similar to this one to test some solutions. Salt water does conduct electricity, but sugar water does not because of the carbon. Gatorade conducts electricity but barely because of the high sugar amount in the drink.
Finally we talked about Molarity. Molarity is moles/Liters and is a quantitative way to measure concentration. Molarity descirbes with numbers if a solution is dilute or concentrated. It is a pretty easy formula so students zoomed through it. Molarity changes with the amount of solute OR the amount of solvent (liquid).
The formula is M1V1=M2V2. These questions will mention molarity twice and volume twice and will not mention moles. Three numbers are given and the fourth needs to be solved for by isolating it through division.
When diluting substances the molarity (concentration) decreases or becomes more dilute because more water is added to the original solution. The number of moles of solute stays the same, but the molarity changes because the volume increases.
The formula is M1V1=M2V2. These questions will mention molarity twice and volume twice and will not mention moles. Three numbers are given and the fourth needs to be solved for by isolating it through division.
When diluting substances the molarity (concentration) decreases or becomes more dilute because more water is added to the original solution. The number of moles of solute stays the same, but the molarity changes because the volume increases.
Tuesday, September 27, 2011
Solutions
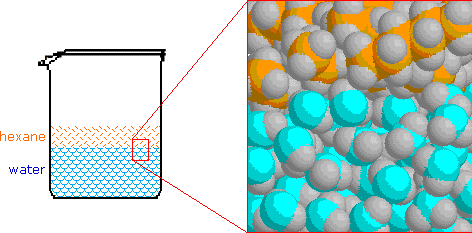
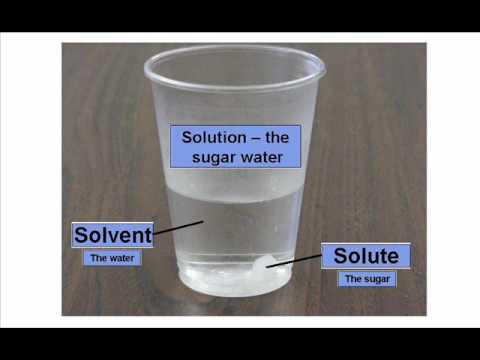
Today in class we discussed solutions. Solutions are homo- geneous mixtures comprised of solutes dispersed in a solvent. Water is the universal solvent, but not the only solvent. For example, a marshmallow is a solid (sugar) solute dispersed in a gaseous solvent (air).
Solubility is how well something dissolves. Some things are very soluble, and some are insoluble (do not dissolve).
Solutions are said to be saturated if they are holding all the solute that they can. When the solute starts to build up on the bottom, you know a solution is definitely saturated (like the dark blue solution on the right). Solutions are unsaturated if they can dissolve more solute (like the two light blue solutions on the left).
Solutions can be super-saturated if they are heated because they can hold more solute than normal. Even if you cool these solutions back down, they will still hold this additional solute in solution. Sweet Tea and all candies are made by first making super-saturated solutions and then cooling them.
For an excellent website about all of these topics and others regarding solutions that have and will be covered in this unit - check out this useful website.
Sunday, September 25, 2011
Matter
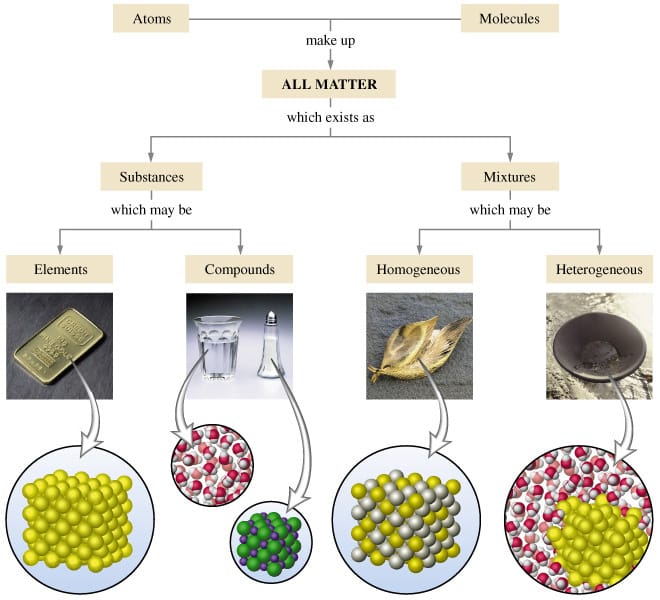
Today we discussed matter. Everything in the universe is made up of matter. Matter can be classified as substances of mixtures. A substance is pure and is either made up of elements, the simplest form of matter found on the periodic table, or compounds - two or more atoms chemically combined that can be represented with a formula (ie. H2O).
A mixture is two or more things (substances or anything really) in the same place at the same time. Salt water is a tricky one because we know we can write a formula for salt (NaCl) and water (H2O), but it is a mixture because when mixed in solution, these compounds do not combine. Mixtures can be heterogeneous of homogeneous based on how the solute is spread through the mixture. Heterogeneous mixtures are not mixed evenly and each sample could be different - like salad, chocolate chip cookie dough ice cream, air, and soil. Homogeneous mixtures are the same throughout like creamy peanutbutter, vanilla ice cream, pure air, sugar, and kool-aid.

One important thing we discussed today was the Law of Conservation of Matter proposed by Lavoisier. Basically matter cannot be created or destroyed... it is conserved or recycled or moved somewhere else, but it cannot magically appear or disappear!
After discussing these ways to classify matter, teams worked on sorting matter. Instead of doing this all at once we did this in four rounds, first asking them to classify substances versus mixtures, then classifying the the substances as elements or compounds, then the mixtures as homogeneous or heterogeneous and finally another mixture sort just to make sure they got it.
A mixture is two or more things (substances or anything really) in the same place at the same time. Salt water is a tricky one because we know we can write a formula for salt (NaCl) and water (H2O), but it is a mixture because when mixed in solution, these compounds do not combine. Mixtures can be heterogeneous of homogeneous based on how the solute is spread through the mixture. Heterogeneous mixtures are not mixed evenly and each sample could be different - like salad, chocolate chip cookie dough ice cream, air, and soil. Homogeneous mixtures are the same throughout like creamy peanutbutter, vanilla ice cream, pure air, sugar, and kool-aid.
One important thing we discussed today was the Law of Conservation of Matter proposed by Lavoisier. Basically matter cannot be created or destroyed... it is conserved or recycled or moved somewhere else, but it cannot magically appear or disappear!
After discussing these ways to classify matter, teams worked on sorting matter. Instead of doing this all at once we did this in four rounds, first asking them to classify substances versus mixtures, then classifying the the substances as elements or compounds, then the mixtures as homogeneous or heterogeneous and finally another mixture sort just to make sure they got it.
Wednesday, August 24, 2011
Tuesday's Earthquake
Class began as normal with students retrieving their homework and starting on two conversion problems. Then the room began to shake. It took me a few moments to register what was happening, then once I realized it was an earthquake I told the students to get under their desks. From what I have read online the shaking lasted about 45 seconds, but anyone who was there knows it felt much longer.
The room was still unsteady when the fire alarms started to go off. I had my students wait a few moments so I could relay information. I had them take their backpacks, cautioned them to be careful, quick, and calm. My students were excellent. It took me a few moments to grab my phone, keys, and name sign and I was one of the last down the stairs. Students were exiting very quickly and taking it well. One girl's backpack fell open and three boys stopped grabbed all her things and kept walking.
My students met me at the bottom of the hill, our prearranged meeting location and sat quietly. I answered any questions I could about earthquakes and what was happening. While we waited in the sunshine we could hear and feel aftershocks. I am very proud of my students for staying calm and sharing phones so that others could try to get in touch with their loved ones.
After the buses had arrived students walked calmly to the bus loop and got on the buses to go home. Students that drove to school and had their keys were allowed to get in their own vehicles and drive home. On the whole, I am amazed how calm everyone was during a situation that does not frequently occur in Virginia.
Currently all buildings are locked and being patrolled by the local authorities so any belongings left by students are safe. When students are allowed to re-enter the building that information will be passed along. Please enjoy your time off until school resumes the Tuesday after Labor Day.
The room was still unsteady when the fire alarms started to go off. I had my students wait a few moments so I could relay information. I had them take their backpacks, cautioned them to be careful, quick, and calm. My students were excellent. It took me a few moments to grab my phone, keys, and name sign and I was one of the last down the stairs. Students were exiting very quickly and taking it well. One girl's backpack fell open and three boys stopped grabbed all her things and kept walking.
My students met me at the bottom of the hill, our prearranged meeting location and sat quietly. I answered any questions I could about earthquakes and what was happening. While we waited in the sunshine we could hear and feel aftershocks. I am very proud of my students for staying calm and sharing phones so that others could try to get in touch with their loved ones.
After the buses had arrived students walked calmly to the bus loop and got on the buses to go home. Students that drove to school and had their keys were allowed to get in their own vehicles and drive home. On the whole, I am amazed how calm everyone was during a situation that does not frequently occur in Virginia.
Currently all buildings are locked and being patrolled by the local authorities so any belongings left by students are safe. When students are allowed to re-enter the building that information will be passed along. Please enjoy your time off until school resumes the Tuesday after Labor Day.
Monday, August 22, 2011
To begin class we went over the weekend's homework and discussed lab techniques.
Next, a few volunteers threw squishy whales at a bullseye. We then discussed who was the closest to the middle and who was the most consistent. This related to accuracy (how close a measurement is to the real measurement) and precision (how close a set of points are to each other) which is what we discussed first. We did some examples as a class and then tied accuracy and precision to percent error.
We practiced calculating percent error by measuring each other's ears and comparing the measurements to the correct value as measured by MsJ.
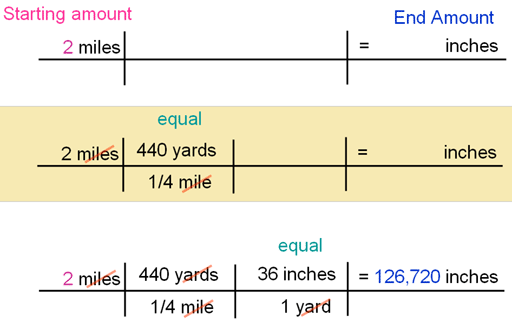
We finished class with some dimensional analysis. Dimensional analysis (or train tracks) is used to convert from one unit to another. Right now we are just learning the process, but these conversions are very important in chemistry and even have their own special name in that regard - stoichiometry.
The important thing to remember in conversions is to match the units diagonally (and usually down) so they cancel and you are able to convert to the new unit you are trying to get to. Some conversions are going to take more than one step. For right now we are doing a mix of standard conversions and completely made up ones.
Anyone who needs help with this needs to let me know! This website has a pretty good explanation.
Anyone who needs help with this needs to let me know! This website has a pretty good explanation.
Homework for this evening is to measure some items around the house using no rulers! We will be doing our first chemistry lab on Wednesday.
Friday, August 19, 2011
Gummy Bear Conclusions
Today students got to see what happened when gummy bears are soaked in water for 24 hours. They get huge! Students could see that the gummy bears were larger, but really had a good idea of the size change when they compared their big bear to a control, a normal size gummy bear.
Morgan's bear did not get bigger because he used the sink with hot water. His gummy bear dissolved!
Some students had difficulties measuring the gigantic bears because now they were more fragile and quite a few lost feet and some bears were just blobs. Students worked on their measurements and writing good conclusions.The gummy bears absorbed water and as a result gained in volume and became less dense because the particles moved further apart.
Some students had difficulties measuring the gigantic bears because now they were more fragile and quite a few lost feet and some bears were just blobs. Students worked on their measurements and writing good conclusions.The gummy bears absorbed water and as a result gained in volume and became less dense because the particles moved further apart.
Thursday, August 18, 2011
Lab Equipment and Gummy Bears
The winners of the lab equipment challenge were Moe, Seth, and Adam. They were given a wordbank (that was honestly incomplete) and a room full of numbered lab equipment and challenged to identify it all.
Today students started a lab involving the scientific method and measuring gummy bears. The students got their bears and measured them and then covered them in water to wait. While waiting students identified lab equipment and took their Thursday Quiz.
 While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.
While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.
Everyone complains they can't eat the bears at the beginning, but complain they don't want to eat them at the end. The cool thing is - there is one more bear in the wings sitting for 24 hours... what is going to happen to it?
Homework was a handout on lab equipment and there is a safety test tomorrow.
Today students started a lab involving the scientific method and measuring gummy bears. The students got their bears and measured them and then covered them in water to wait. While waiting students identified lab equipment and took their Thursday Quiz.
 While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.
While we were working, the bears absorbed water and expanded in size. They get bigger and gooey-er and slimy and it's fun for me to watch. Morgan used hot water in his cup and his bear dissolved and shrank into a skele-bear.Everyone complains they can't eat the bears at the beginning, but complain they don't want to eat them at the end. The cool thing is - there is one more bear in the wings sitting for 24 hours... what is going to happen to it?
Homework was a handout on lab equipment and there is a safety test tomorrow.
Wednesday, August 17, 2011
First Week - Density and Safety
Monday was a get-to-know-you sort of day. Students started with an informal survey and drew a picture of themselves that I will use to help learn their names (I am terrible at names). I familiarized the students with classroom procedures, discussed homework and classwork, and let them know how class was going to run.
We have made sure that everyone had a good handle on density in regards to definition, math and formulas, and what it actually means. Density is how close together the particles are in a substance. If they are close together the substance is more dense. If the particles are far apart, the substance is less dense. I do not float in Lake Anna, but I do float in the ocean - therefore I am more dense than Lake Anna and less dense than the ocean.
Students made predictions for a density column including corn syrup, water, mouthwash, baby oil, and vegetable oil. Things that are more dense-sink, things that are less dense-rise to the top, things with similar densities-mix. Then I poured the liquids in a randomly asked for order and students looked at how the layers formed because of the differences in density. They also fielded oral questions as the demonstration was performed. Here is a photo of a similar demo.
Today we are going to cover lab equipment and do a fun gummy bear lab. Here is a good handout to review them. Students will try on their own to id the equipment before I tell them what everything is.
Last night's homework was box A&B on the blue sheet. Tonight it is box C&D. Tomorrow is Thursday so there is a syllabus/density quiz and the safety test will be on Friday.
Tuesday, August 9, 2011
Welcome Back! Fall 2011
Greetings students, parents, and guardians.
Welcome to a new school year with Ms Jancaitis! This blog has been set up to connect students, parents, and guardians with the chemistry class.
At Open House or in class, each student will receive a course syllabus, safety rules, and a breakage sheet. The safety rules and breakage sheet needs to be read and signed by both the student and parent guardian.
Welcome to a new school year with Ms Jancaitis! This blog has been set up to connect students, parents, and guardians with the chemistry class.
At Open House or in class, each student will receive a course syllabus, safety rules, and a breakage sheet. The safety rules and breakage sheet needs to be read and signed by both the student and parent guardian.
- The course syllabus outlines what the course will be like, what topics will be covered, and course expectations. It also contains contact information. There will be a quiz on this syllabus on Thursday.
- The safety rules are rules designed to keep the classroom safe and orderly to maximize learning and prevent accidents and injuries. These rules need to be studied because there will be a safety test on Friday and infractions of these rules can lead to disciplinary action as well as low assignment grades.
- A breakage sheet is a contract holding students accountable for the items that are broken if the student is acting a manner that is unsafe for themselves or those around them.
Monday, May 30, 2011
Congrats and Good Luck!
Congratulations to all of LCHS's spring chemistry students for passing the end of course assessment.
Congratulations to LCHS's class of 2011.
Good luck and best wishes to 2011 seniors - to everyone else, I'll see y'all in the fall :)
Friday, April 29, 2011
acids and bases
Today we started discussing acids and bases.
If you mix an acid and a base together, then there is a neutralization reaction. A neutralization reaction is also a double replacement reaction. In neutralization, the acid and base combine to form a water and a salt. The water and the salt are neutral (hence the name).
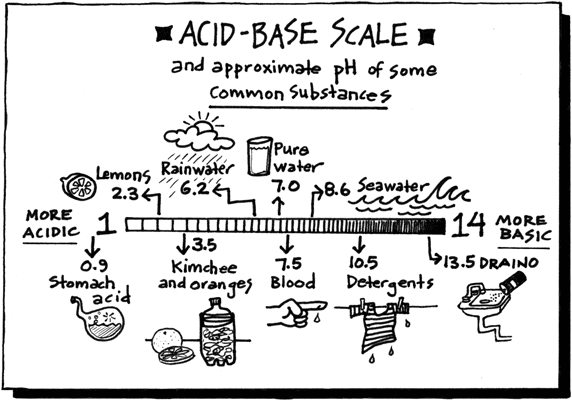
pH measures the concentration or molarity of H (Hydrogen ions) in a solution. That's why the H is capitalized.
pH + pOH = 14. So if you have the pH, it is easy to get the pOH... just subtract from 14. There are some fun interactives for acids and bases linked at the bottom of this webpage. Check out the Alien Juice Bar.
Acids have H+ and donate them, they have the smaller numbers 0-6.9, they turn litmus red, and have sour tastes.
Bases have OH- and want H+ to make water, they have Bigger numbers, they have a pH between 7.1 and 14, they turn litmus Blue, and they taste Bitter.
If you mix an acid and a base together, then there is a neutralization reaction. A neutralization reaction is also a double replacement reaction. In neutralization, the acid and base combine to form a water and a salt. The water and the salt are neutral (hence the name).
pH measures the concentration or molarity of H (Hydrogen ions) in a solution. That's why the H is capitalized.
pH + pOH = 14. So if you have the pH, it is easy to get the pOH... just subtract from 14. There are some fun interactives for acids and bases linked at the bottom of this webpage. Check out the Alien Juice Bar.
Tuesday, April 26, 2011
Combined Gas Law

The combined gas law combines the work of Charles, Boyle, and Gay-Lussac.
PV = PV
nT nT
Basically, memorize one formula and then use only the variables you need, so sometimes you need PV = PV, and sometimes V/T = V/T.
This will help you with placement and deciding whether you should multiply or divide.
Sunday, April 24, 2011
Ideal Gas Law
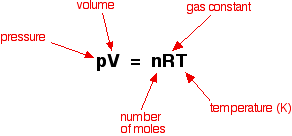
Ideal gases do not actually exist, but we pretend they do and use the Ideal Gas Formula of PV=nRT.
One of these variables will not be given to you and you have to solve for it. This does not seem difficult after stoich, so students dove in, did well, and finished early.
Saturday, April 23, 2011
Beginning Gases
Today we started learning about the behavior of gases and the factors that affect them. Gases are lightweight fast moving particles that generally have a lot of empty space between them. Because of this, they are easily compressible (pictured left). If not contained, gases can spread (or diffuse) to fill any size and shape container.
 Gases are affected by pressure, volume, number of moles, and temperature. Changing any one of these variables, changes all the others.
Gases are affected by pressure, volume, number of moles, and temperature. Changing any one of these variables, changes all the others.
Today we also learned the formula for the Law of Partial Pressure. Basically partial pressures add up to form total pressure. If the total pressure is given then you subtract the partial pressures.
We are still working on moles and making sure we have stoichiometry down pat.
 Gases are affected by pressure, volume, number of moles, and temperature. Changing any one of these variables, changes all the others.
Gases are affected by pressure, volume, number of moles, and temperature. Changing any one of these variables, changes all the others.Today we also learned the formula for the Law of Partial Pressure. Basically partial pressures add up to form total pressure. If the total pressure is given then you subtract the partial pressures.
We are still working on moles and making sure we have stoichiometry down pat.
Thursday, April 21, 2011
Gum Lab
Students each had a piece of gum and observed the gum by weighing it, drawing it, and smelling it. All the gum was Dubble Bubble, but the Cry Babies were the most popular.
Next the students chewed the gum for ten minutes. While they were waiting we watched How Its Made on bubblegum (the video is linked on the side if you want to check it out). We also had a little fun blowing bubbles.
After ten minutes, students did more observations and re-weighed the gum. The gum weighed less... why? Because the sugar dissolved and was lost. Using this weight difference, students determined the percent composition of sugar in the gum they chewed. They also can convert the grams to moles and determine how many moles of sugar were in the gum.
Next the students chewed the gum for ten minutes. While they were waiting we watched How Its Made on bubblegum (the video is linked on the side if you want to check it out). We also had a little fun blowing bubbles.
After ten minutes, students did more observations and re-weighed the gum. The gum weighed less... why? Because the sugar dissolved and was lost. Using this weight difference, students determined the percent composition of sugar in the gum they chewed. They also can convert the grams to moles and determine how many moles of sugar were in the gum.
Monday, April 18, 2011
MOLES
Today students took a mole challenge to see how much they knew about moles... besides their favorite number. The high score was a 9.5/10 so I must have stumped them. They did do better than they thought they would... and most of them were a bit mad when they saw what the answers were to the ones they didn't know.
For moles there are basically three conversions to know.
1 mole
6.02 x 10^23
1 mole(molar mass) grams (add weights from PT)
1 mole
22.4 Liters
Today we learned about Avogadro's theory about gases and then practiced converting using our new favorite number, 22.4. Avogadro, that handsome devil, did a lot of important stuff with moles and is also the guy who gave us our other favorite number 6.02.
For moles there are basically three conversions to know.
1 mole
6.02 x 10^23
1 mole(molar mass) grams (add weights from PT)
1 mole
22.4 Liters
Today we learned about Avogadro's theory about gases and then practiced converting using our new favorite number, 22.4. Avogadro, that handsome devil, did a lot of important stuff with moles and is also the guy who gave us our other favorite number 6.02.
Sunday, April 17, 2011
What is a mole?
Today, we started talking about moles. Moles are used to count atoms. There are 22,000,000,000,000,000,000 quintillion atoms in a grain of sand and even counting grains of sand is a pain. Because atoms are so tiny, we use the mole to estimate.
There are 6.02 x 10 ^23 molecules in one mole. That's a whole lot. This is our new favorite number because it needs to be memorized. We practiced converting from moles to molecules.
Next we discussed molar mass. Molar mass = 1 mole and it also equals atomic mass from the periodic table. To find the molar mass of carbon dioxide you find the mass of carbon and two oxygens and add them together. Finding molar mass is not difficult unless the molecule has tricky subscripts (which we have been practicing).
There are 6.02 x 10 ^23 molecules in one mole. That's a whole lot. This is our new favorite number because it needs to be memorized. We practiced converting from moles to molecules.
Next we discussed molar mass. Molar mass = 1 mole and it also equals atomic mass from the periodic table. To find the molar mass of carbon dioxide you find the mass of carbon and two oxygens and add them together. Finding molar mass is not difficult unless the molecule has tricky subscripts (which we have been practicing).
Saturday, March 26, 2011
Reaction Rate Basics
Reaction Rates are affected by a few things. Without telling them the point, the students had a quick demo where they had to dissolve sugar cubes the fastest.
The things that speed up reactions are:
- Temperature - warmer is faster
- Surface Area - small pieces have more surface area
- Concentration - the more water, the faster sugar will dissolve
- Catalyst - lowers the activation energy and speeds up the reaction
- Agitation - shaking or stirring increases the frequency of collisions.
Le Chatlier
Students learned about reaction rates and how to increase them. They also learned about reversible reactions and how Le Chatlier's principle influences shifts of equilibrium in reversible reactions.
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
 Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
 Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).
Pressure is the tricky one. If pressure is applied to an equilibrium, then the reaction will shift to the side that has the least amount of molecules (count the coefficients).