Friday, February 27, 2015
Chemistry Of: Beets and Asparagus
Of course, you need a video about Asparagus Pee! Check out this one from the SciShow!
This is a very informative, powerful TEDtalk about human waste and why sanitation is important.
The original Compound Interest post on Asparagus can be found here; the article on Beets can be found here. And if the beet thing has never happened to you - you can google it to see images, just remember what you are looking up so you know what to expect. ;)
Thursday, February 26, 2015
Insect Pain Scale update
 I was listening to a RadioLab podcast today and it was about the insect pain scale! The whole podcast is about pain, but the first ten minutes are an interview with the dude Schmidt who developed the scale. Pretty neat! You can listen to it here.
I was listening to a RadioLab podcast today and it was about the insect pain scale! The whole podcast is about pain, but the first ten minutes are an interview with the dude Schmidt who developed the scale. Pretty neat! You can listen to it here.
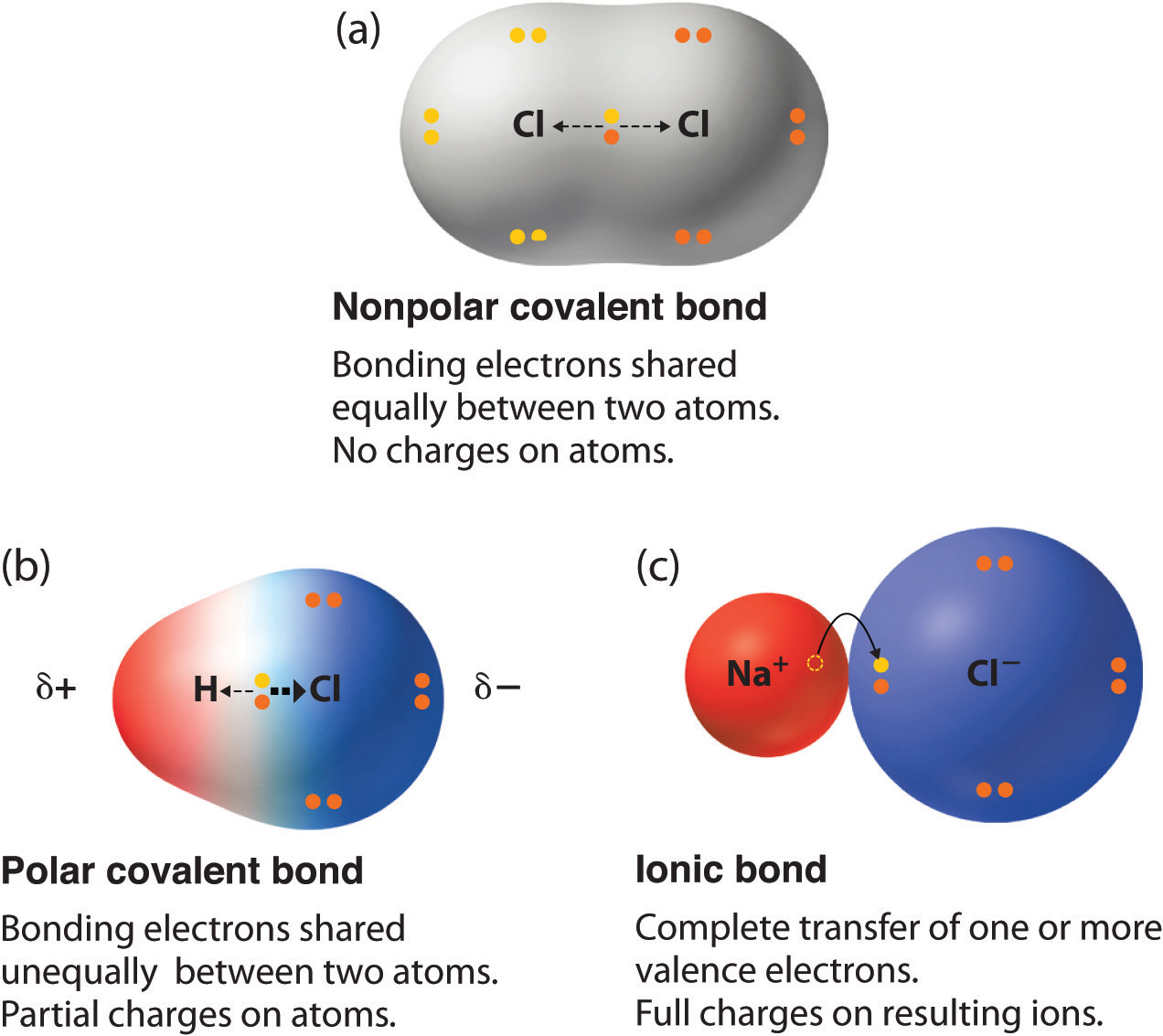
Polar vs NonPolar Covalent Bonds
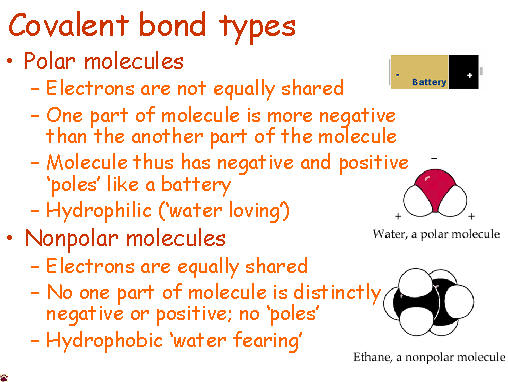 Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different.
Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different.
If a molecules is nonpolar covalent, it is sharing its electrons equally. The best example of this is in diatomic molecules. Diatomic molecules are two of the same atom bonded together - so they would have exactly the same pull. Symmetrical molecules are also nonpolar.
Polar covalent bonds occur when electrons are not equally shared. One atom, usually more electronegative, has a stronger pull on the electrons and shares them unequally. The other atom that is less electronegative has a smaller hold on the electrons and is thus can be slightly positive.
One way to remember this is... "Polar Bears do not share... equally."
Tuesday, February 24, 2015
VSEPR
Valence Shell Electron Repulsion Theory
Electrons do not like each other and when looking at molecular structures - electrons and unshared electrons (the two dots paired together) will space out evenly so they are as far apart as possible.
Most of the names of the shapes of hints like tri, tetra, planar, etc. Students need to memorize these shapes and be able to visualize them for given formulas.
For help with VSEPR - read this.
Electrons do not like each other and when looking at molecular structures - electrons and unshared electrons (the two dots paired together) will space out evenly so they are as far apart as possible.
Most of the names of the shapes of hints like tri, tetra, planar, etc. Students need to memorize these shapes and be able to visualize them for given formulas.
For help with VSEPR - read this.
Tuesday, February 17, 2015
Covalent Bonding
If it is a - and -, the bond is covalent. The electrons are shared in the bond. To get the formula, you have to draw the Lewis Dot structures for the elements and connect the dots that don't have friends. You write the formula based on your drawing. To name it, use prefixes to indicate the number of atoms in the formula and the second one ends in -ide. For these it doesn't matter which element comes first.
There are 4 atoms that commonly form diatomic molecules with a covalent bond... and hydrogen is one of them! That weird atom!
Saturday, February 14, 2015
Friday, February 13, 2015
Metals = Roman Numerals
 Time to learn about roman numerals.... Here is a handy clock if you are unfamiliar with them. Pretty much you need to know 1-7. 1 is represented with I, five with V and 10 with X. 4 and 6 and 7 is where it gets tricky. 4 is 1 before 5 - so its Roman numeral is IV. 6 is one after five so its roman numeral is VI.
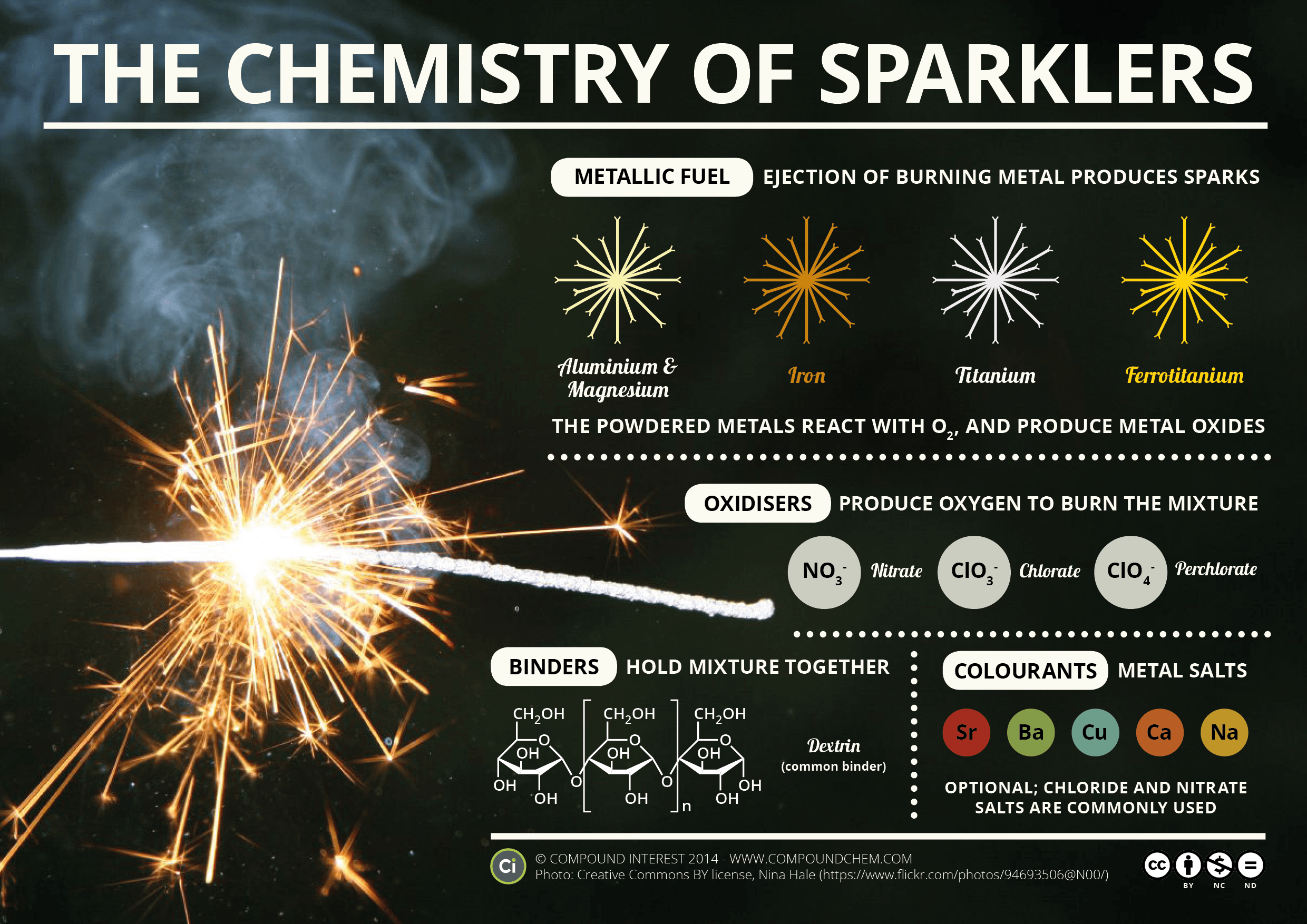
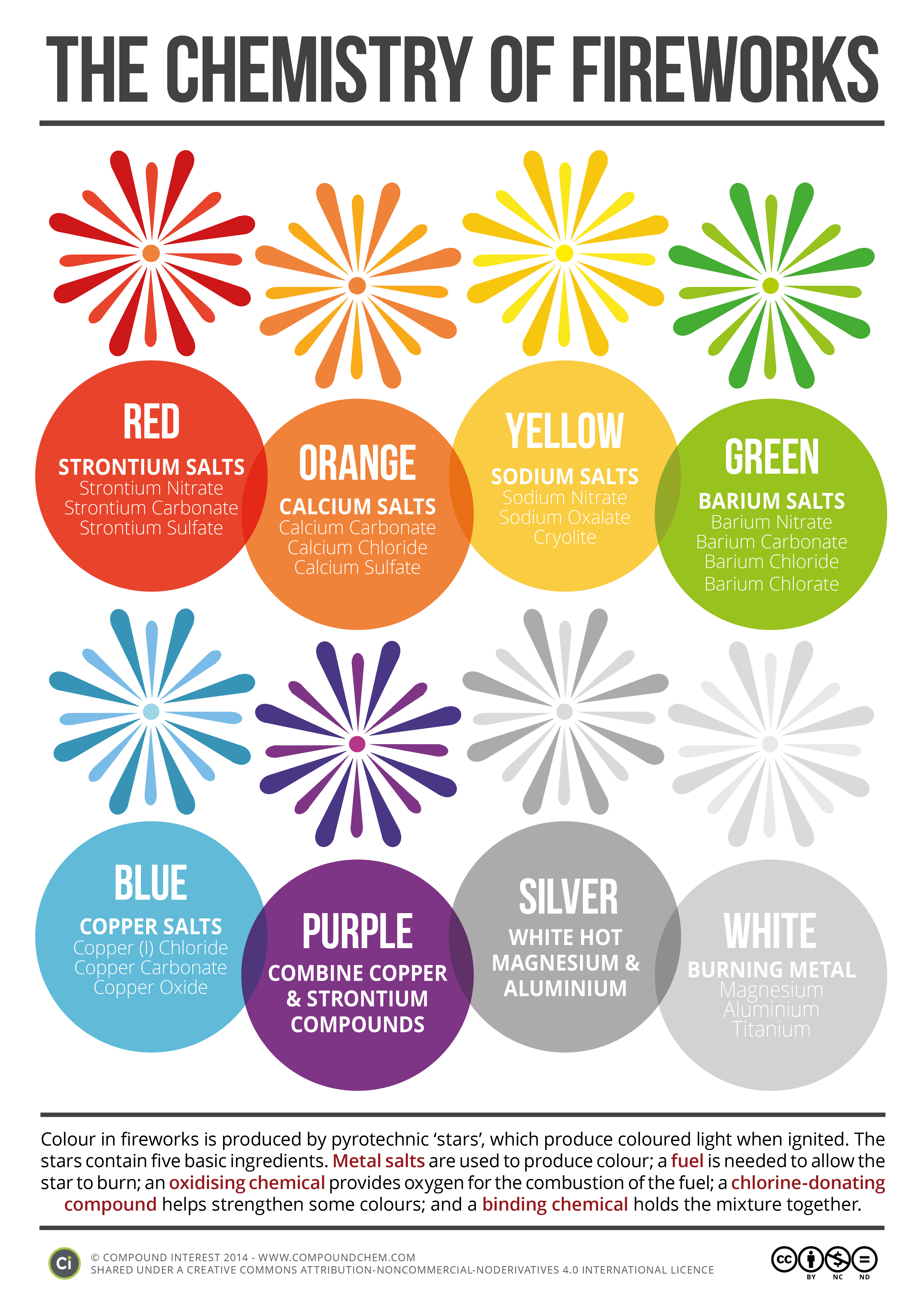
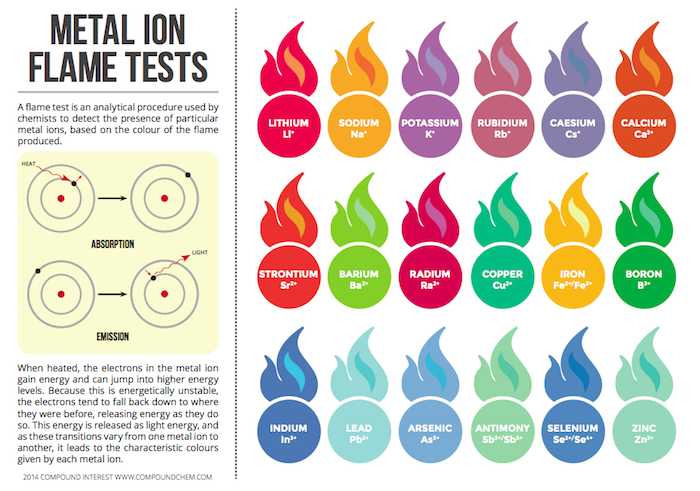
Time to learn about roman numerals.... Here is a handy clock if you are unfamiliar with them. Pretty much you need to know 1-7. 1 is represented with I, five with V and 10 with X. 4 and 6 and 7 is where it gets tricky. 4 is 1 before 5 - so its Roman numeral is IV. 6 is one after five so its roman numeral is VI.Chemistry Of: Metal Ions, Sparklers, and Fireworks!
The original image is HERE.
These images are originally from Compound Interest. They can be found at: Sparklers, Fireworks, and Metal Ions.
This TedEd video relates all of this the Chemistry Dudes like DeBroglie!
Want more? Check out these two videos from ScienceFriday about "The Science of Fireworks" and "Celebrating Explosive Chemistry."
Thursday, February 12, 2015
Ionic Bonding
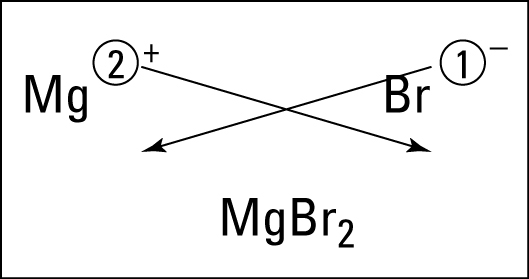
 Students learned about ionic bonding. Ionic bonding happens between metals and nonmetals (positives and negatives). The electrons are given and taken in this ionic bond.
Students learned about ionic bonding. Ionic bonding happens between metals and nonmetals (positives and negatives). The electrons are given and taken in this ionic bond. To get the formula, you criss cross the charges. To name it, you say the name of the metal, then the name of the nonmetal with an -ide ending. If it is a metal from DForP block, then you use a roman numeral to indicate the charge of the metal. Basically everyone gets a Roman numeral except S-block, Boron, and Aluminum
 After learning the basics, students practice.
After learning the basics, students practice. Now that we understand ionic bonding, students should find this cartoon amusing.
Now that we understand ionic bonding, students should find this cartoon amusing.Ionic Bonds for Dummies
Here is a cool interactive where you can build models to simulate ionic bonding.
Wednesday, February 11, 2015
Valence Electrons and Charges of Ions
Students learned about valence electrons. Valence electrons are the outermost electrons and are the electrons that are used for bonding and participate in reactions. Valence electrons are only found in the S and P blocks. The max number of valence electrons is 8. Students practiced counting valence electrons and drawing Lewis Dot Structures.
Students also practiced identifying which noble gas an element wanted to be like. All elements want to be like two noble gases - it is just a matter of figuring out which is closer. Elements want to be like noble gases because they have full outer electron shells, or full valences. This makes them stable and non reactive which is why noble gases are sometimes called the inert gases.
Today students learned how to use valence electrons and dot structures to determine the charge of an atom. Atoms either want to gain electrons or lose electrons to become like those noble gases they envy.
Students also practiced identifying which noble gas an element wanted to be like. All elements want to be like two noble gases - it is just a matter of figuring out which is closer. Elements want to be like noble gases because they have full outer electron shells, or full valences. This makes them stable and non reactive which is why noble gases are sometimes called the inert gases.
Today students learned how to use valence electrons and dot structures to determine the charge of an atom. Atoms either want to gain electrons or lose electrons to become like those noble gases they envy.
- Ions are atoms or molecules that have a net charge, either positive or negative. There are two kinds of ions:
- Anions are negatively charged ions because they have negative net charges. This means that there is a greater number of electrons (-) than protons (+). For example, the anion, fluoride (F 1-), has a one negative charge because it has a total of nine protons and ten electrons. Thus, the net charge for fluoride is 1 negative.
- Cations are positively charged ions because they have positive net charges. This is due to these ions having more protons (positive charges) than electrons (negative charges). For example, calcium (Ca 2+) is a cation ion with 20 protons and 18 electrons. The net charge for Calcium is 2 positive. (from here)
Tuesday, February 10, 2015
Formula Writing
 Today we discussed how to write chemical formulas, and what the numbers associated with a chemical formula mean.
Today we discussed how to write chemical formulas, and what the numbers associated with a chemical formula mean.Coefficients are the big numbers in front and are distributed to the whole molecule (which means you may have to multiply). Coefficients tell you how many molecules are present.
3He = He He He :)
Subscripts are the little lower numbers and they indicate the number of atoms and only apply to the atom it is to the right of. Subscripts tell you how many of each atom are present. Students wrote their name as a chemical compound and thought it looked pretty interesting. Some students have long formulas, other short.
We then led into counting atoms for real chemical formulas using subscripts and coefficients.
Be careful....
Here is the wonderful website these images came from. You may find it helpful.
Wednesday, February 4, 2015
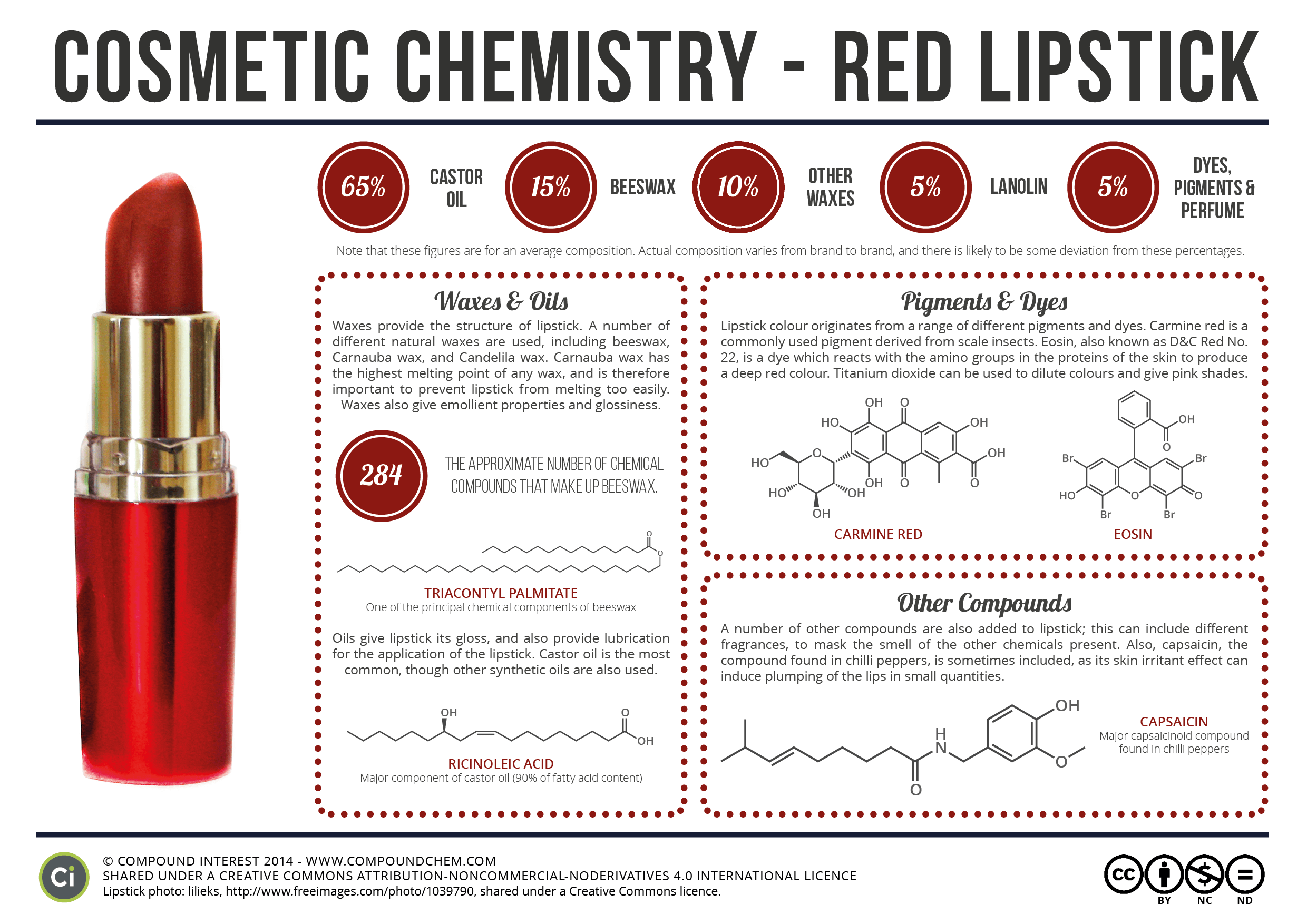
Chemistry of: Red Lipstick and Chocolate
Compound Interest's original post about lipstick can be found here. Compound Interest's original post about chocolate can be found here. Compound Interest's original post about the structure of chocolate can be found here.
For more on the chemistry of makeup, check out this article from HowStuffWorks and this one from Beauty By the Geeks.
It's not exactly chocolate, but here is a TedEd talk on the chemistry of baking cookies. Yum!
Check out Food Network's show Unwrapped to see how some chocolate candies are made. Do you know your chocolate candy bars?
Try this online quiz on chocolate bar cross sections. MsJ got 7 correct... but there are some less common chocolate bars included.
MsJ and Mrs. Woodward both love anything dark chocolate peppermint!
Tuesday, February 3, 2015
Periodic Trends
It is all about the electrons! Always!
Electro- negativity is how badly atoms want electrons. The most electronegative atoms are Fluorine, Chlorine, and Oxygen. Everyone wants to be a Noble Gas... and halogens are the closest so they are the most electronegative.
Ionization energy is how difficult it is to remove electrons. It is difficult to remove electrons from atoms that are electronegative.
Atomic radius increases as you move down the periodic table because atoms have more mass, but actually decreases from left to right because atoms are holding on to their electrons tighter (because they are more electronegative).
Shielding has to do with how protons are blocked by the electron shells - the more shells there are, the more blocking there is. So something in period 5 (with 5 shells) has more shielding than an element in period 2 with two electron shells. Sheilding is constant across the periodic table because the number of shells is constant.
Monday, February 2, 2015
Electronic Configuration
 Battleship, a classic game by Milton Bradley, is a game easily adaptable to learning electronic configuration.
Battleship, a classic game by Milton Bradley, is a game easily adaptable to learning electronic configuration.Electronic Configuration is an intense mathematical calculation proposed by Schrodinger & Heisenberg as a way to predict where to find an electron around the nucleus in the electron cloud model.
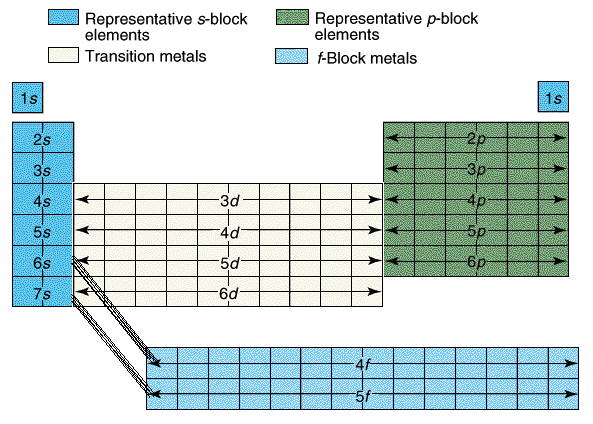
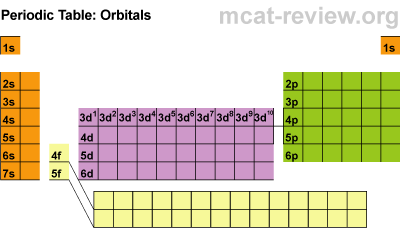
There are four main parts of the periodic table known as orbitals. The S block, P block, D and F orbitals. Within each block, you just count over how many spaces it is. There are seven energy levels that are loosely based on the period that an element is (the D & F blocks are exceptions to energy levels). The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
So to identify Hydrogen you would say 1s2 because it is in the first period or first energy level, in the s block, and the first member of the first block. Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
Students learned the pattern of electronic configuration and how to use it. Basically its like giving directions to an element on the PT using set landmarks. It is a bit confusing, but once you get the pattern, its not too bad.
Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.