 Students learned about ionic bonding. Ionic bonding happens between metals and nonmetals (positives and negatives). The electrons are given and taken in this ionic bond.
Students learned about ionic bonding. Ionic bonding happens between metals and nonmetals (positives and negatives). The electrons are given and taken in this ionic bond. To get the formula, you criss cross the charges. To name it, you say the name of the metal, then the name of the nonmetal with an -ide ending. If it is a metal from DForP block, then you use a roman numeral to indicate the charge of the metal. Basically everyone gets a Roman numeral except S-block, Boron, and Aluminum
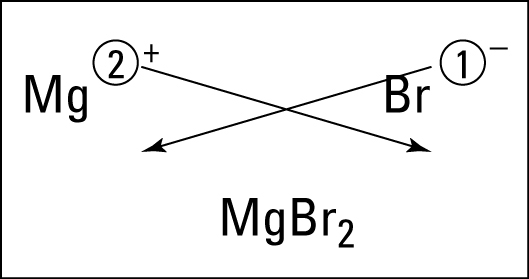 After learning the basics, students practice.
After learning the basics, students practice. Now that we understand ionic bonding, students should find this cartoon amusing.
Now that we understand ionic bonding, students should find this cartoon amusing.Ionic Bonds for Dummies
Here is a cool interactive where you can build models to simulate ionic bonding.

No comments:
Post a Comment
Thanks for your comments :)