 Battleship, a classic game by Milton Bradley, is a game easily adaptable to learning electronic configuration.
Battleship, a classic game by Milton Bradley, is a game easily adaptable to learning electronic configuration.Electronic Configuration is an intense mathematical calculation proposed by Schrodinger & Heisenberg as a way to predict where to find an electron around the nucleus in the electron cloud model.
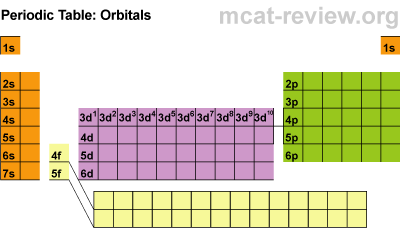
There are four main parts of the periodic table known as orbitals. The S block, P block, D and F orbitals. Within each block, you just count over how many spaces it is. There are seven energy levels that are loosely based on the period that an element is (the D & F blocks are exceptions to energy levels). The D block is dumb and that's why it starts with one number lower. Really they just have less energy and have the same amount of energy as the S and P block in the 3rd period. The F block are failures and that's why they are 2 lower... or they have a lot less energy.
So to identify Hydrogen you would say 1s2 because it is in the first period or first energy level, in the s block, and the first member of the first block. Carbon is a 2P2 because it is in the 2nd period, in the P block, and the 2nd one over in the P block.
Students learned the pattern of electronic configuration and how to use it. Basically its like giving directions to an element on the PT using set landmarks. It is a bit confusing, but once you get the pattern, its not too bad.
Students practiced a bit and then they played Battleship to practice some more. The Periodic Table became the game board and students hid their ships on it, then guessed hits using the electronic configuration of the atoms. I think they really got the hang of it because I did not field many questions at that point.
No comments:
Post a Comment
Thanks for your comments :)