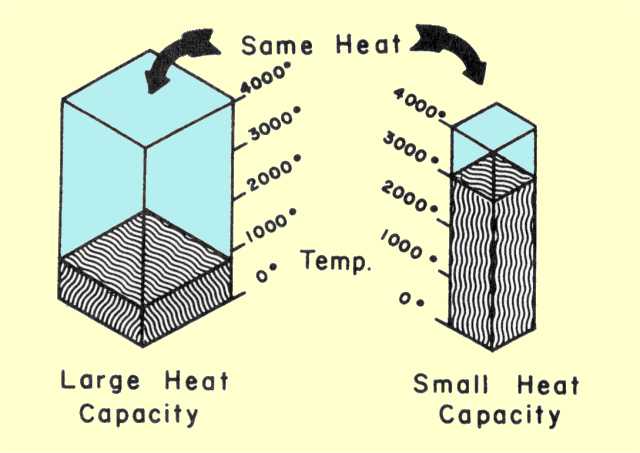
 Heat capacity is the amount of energy needed to raise a substance 1*C.
Heat capacities depend on the amount of the liquid and how it is
contained.
Heat capacity is the amount of energy needed to raise a substance 1*C.
Heat capacities depend on the amount of the liquid and how it is
contained.
Specific heat is the amount of heat needed
to raise 1 gram of a substance 1*C. Specific heat is measured using a
formula with J/gC. (Apparently that now stands for Jancaitis gone crazy
instead of Joules per grams Celsius.)
We
re-learned endo and exothermic reactions.We discussed them in the
reaction unit, but are revisiting the topic because now we are also
going to be calculating heat changes and specific heat values.
Endothermic reactions absorb heat and get warmer (End Up).
Exothermic reactions lose or release heat and get colder (Exit down).
To
test this out, students in groups were given a whack-a-pack and asked
to make observations. The pack starts off at room temperature and when
you hit it, the reaction occurs. This is a chemical reaction for a few
reasons - one you can hear it fizzing. Two it blows up so a gas is being
formed (1 of the 4 ways you know a chemical reaction has occurred). And
Three there is a temperature change (another of the four ways). The
pack gets really cold which means it is releasing heat and this is an
exothermic reaction.
Watch this little video to see how it works. These are available at Dollar
Tree at Valentine's Day if you are interested.
After this lab demo, students answered questions and then worked on math practice for heat changes and specific heat.

No comments:
Post a Comment
Thanks for your comments :)