 For notes today we discussed vapor pressure and boiling point. The
boiling point of a liquid is when the vapor pressure equals the external
pressure. When the pressures are equal it is easier for liquids to boil
and vaporize into gases and steam away. We discussed definitions and
answered questions about vapor pressure graphs. STP is 101.3kpa, 1 atm, or 760 torr.
For notes today we discussed vapor pressure and boiling point. The
boiling point of a liquid is when the vapor pressure equals the external
pressure. When the pressures are equal it is easier for liquids to boil
and vaporize into gases and steam away. We discussed definitions and
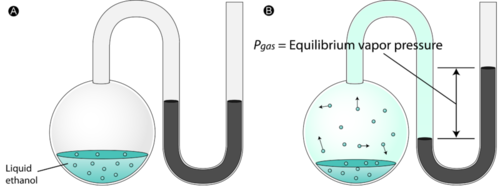
answered questions about vapor pressure graphs. STP is 101.3kpa, 1 atm, or 760 torr.Vapor pressure is measured with a manometer. A "normal" manometer is when the levels in the U are even. A "HOT" manometer has increased vaporization and increased particle movement so the liquid levels in the U are pushed away.
No comments:
Post a Comment
Thanks for your comments :)