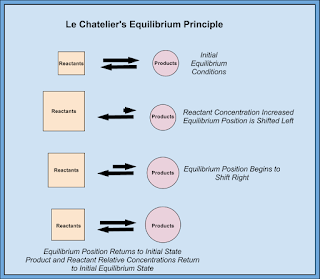
Basically as you apply a stress to a system, the system will shift in response to the stress. If you add one of the molecules it will shift away from that molecule. If you take away a molecule, it will shift towards it to make more. Heat works the same way.
Upwelling
-
Today in class we discussed water temperature and upwelling. For notes we
continued talking about water movement and addressed ocean temperature and
locat...
8 years ago





No comments:
Post a Comment