 After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods.
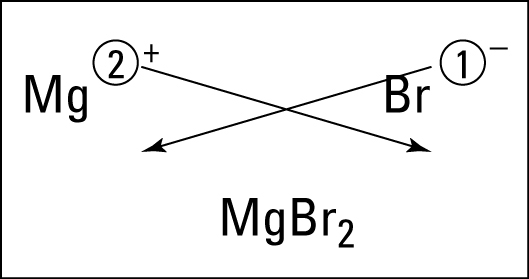
After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods. Now that we understand ionic bonding, students should find this cartoon amusing.
Now that we understand ionic bonding, students should find this cartoon amusing. Ionic Bonds for Dummies
Here is a cool interactive where you can build models to simulate ionic bonding.









No comments:
Post a Comment