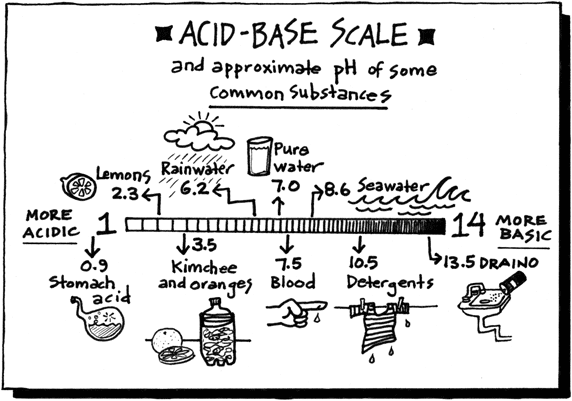
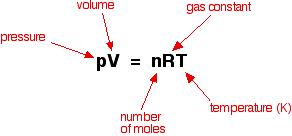
Today you will be completing a practice SOL test. You will do this on this computer. You will need a PT, a calculator, and scrap paper. Answer the questions as best you can and use good test taking skills.
DO NOT go back and DO NOT start over. Instead read the questions carefully and make good choices. If you get a question incorrect I will explain it to you, but you need to try the question on your own. Be patient.
Take your time. At the end of the test you will SHOW me your score. If you do not finish, you will show me how many questions you answered and your score. I will record this as a grade.
 Tomorrow and Friday you will have other assignments on the computer. You will need to come to to this website, read the post, and follow the directions. You will have directions and various scores to report and turn in. Anyone using a computer inappropriately will be reported and computer privileges will be removed.
Tomorrow and Friday you will have other assignments on the computer. You will need to come to to this website, read the post, and follow the directions. You will have directions and various scores to report and turn in. Anyone using a computer inappropriately will be reported and computer privileges will be removed. Ready for you test. Use this link and get started.