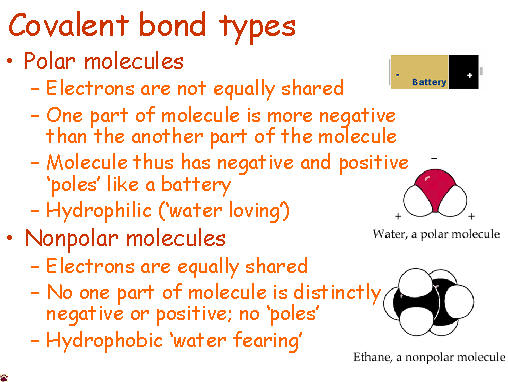
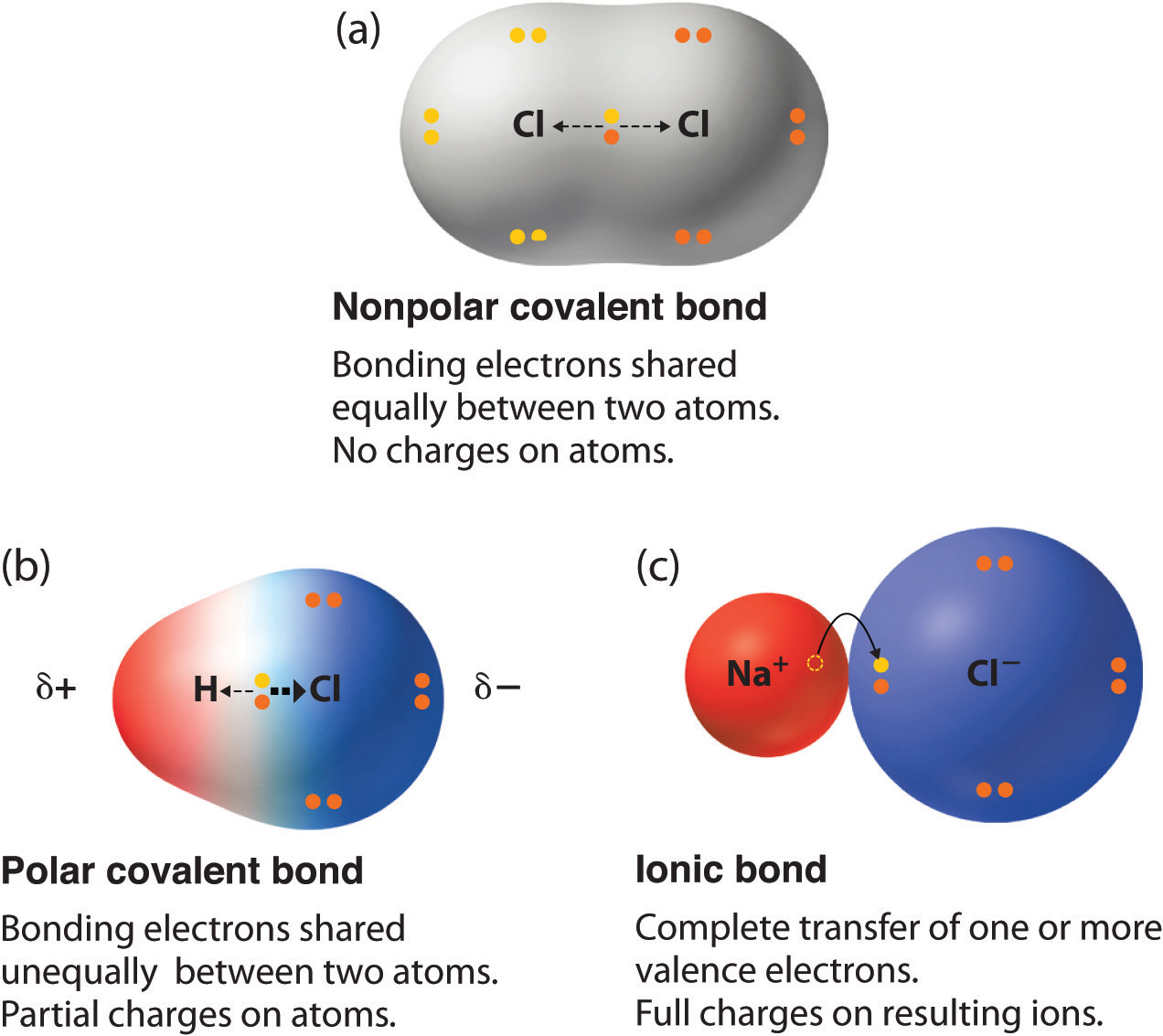
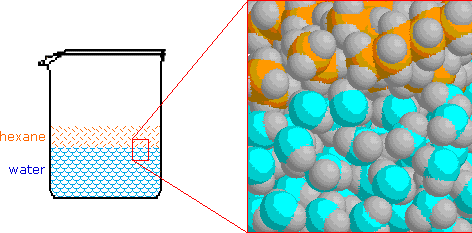
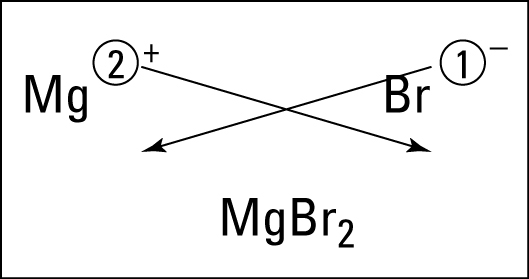
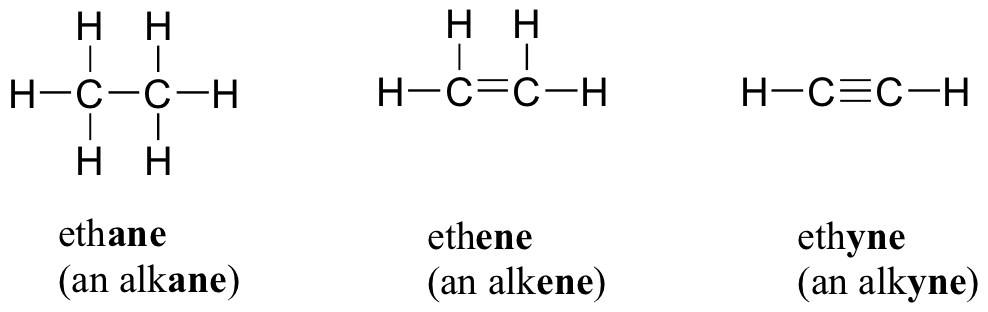
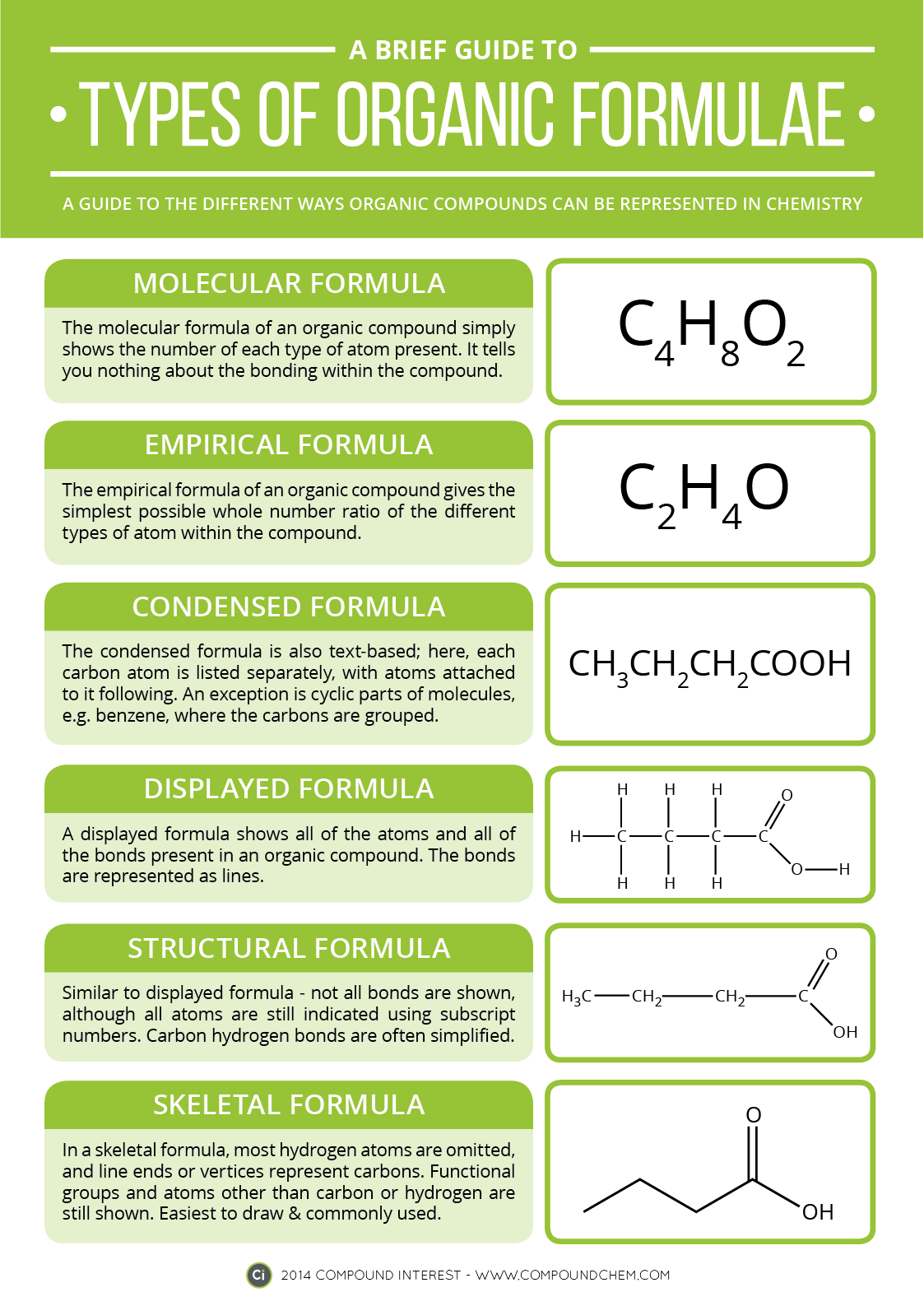
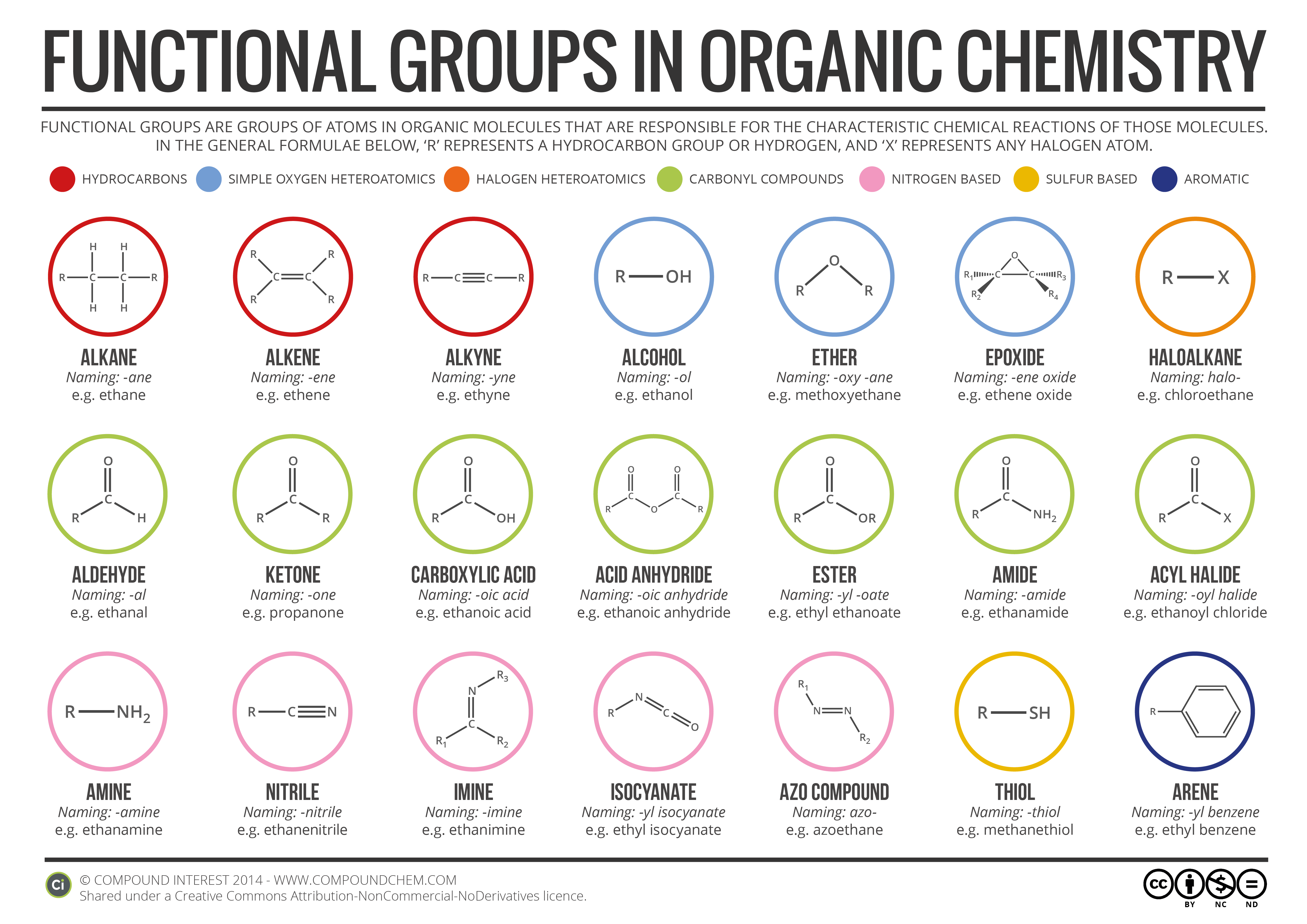
Electrons do not like each other and when looking at molecular structures - electrons and unshared electrons (the two dots paired together) will space out evenly so they are as far apart as possible.
Most of the names of the shapes of hints like tri, tetra, planar, etc. Students need to memorize these shapes and be able to visualize them for given formulas.
For help with VSEPR - read this.