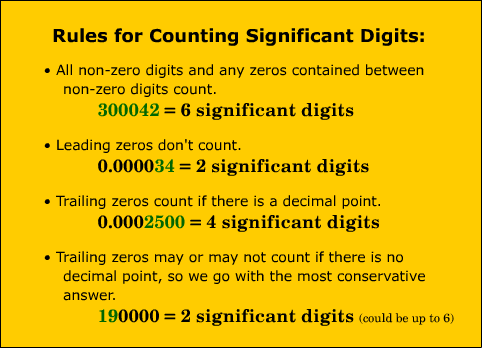
Today students began class with a jump in about the phases of matter. Once we went over the jump in, we began going over the homework from all the snow days. Students did an excellent job of getting their work done and did well on it. We went over it in bits and then gave them a chance to go back and refine their answers. Congratulations to the winners of the Physical/Chemical challenge.
Today we made sure that everyone had a good handle on density in regards to definition, math and formulas, and what it actually means. I do not float in Lake Anna, but I do float in the ocean - therefore I am more dense than Lake Anna and less dense than the ocean.
Students made predictions for a density column including corn syrup, water, mouthwash, baby oil, and vegetable oil. Things that are more dense-sink, things that are less dense-rise to the top, things with similar densities-mix. Then I poured the liquids in a randomly asked for order and students looked at how the layers formed because of the differences in density. They also fielded oral questions as the demonstration was performed. Here is a photo of a similar demo.
Students received grade sheets to check their progress and get missing work turned in. Tonight's homework is a Density Practice Sheet. If tomorrow is a snow day, then students should work on the "mixing solutions" sheet. Both are half sheets.
There will be a quiz this week, Thursday or not.
Global Ocean Conveyor
-
Today students began class with a BrainPop on currents and took the quiz.
Tim and Moby did an excellent job describing how currents affect global
weather ...
6 years ago