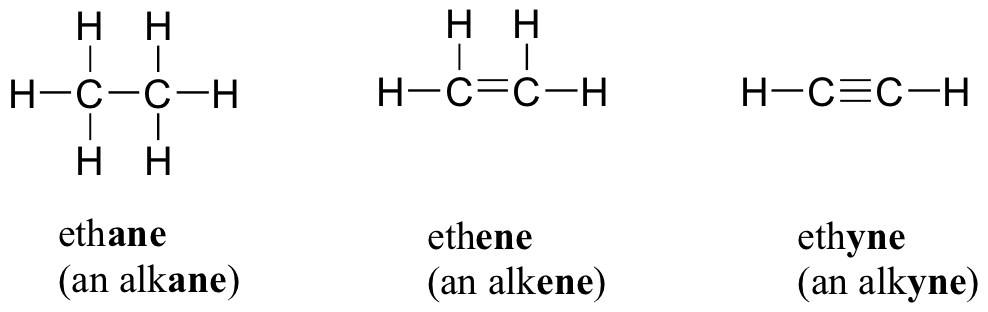
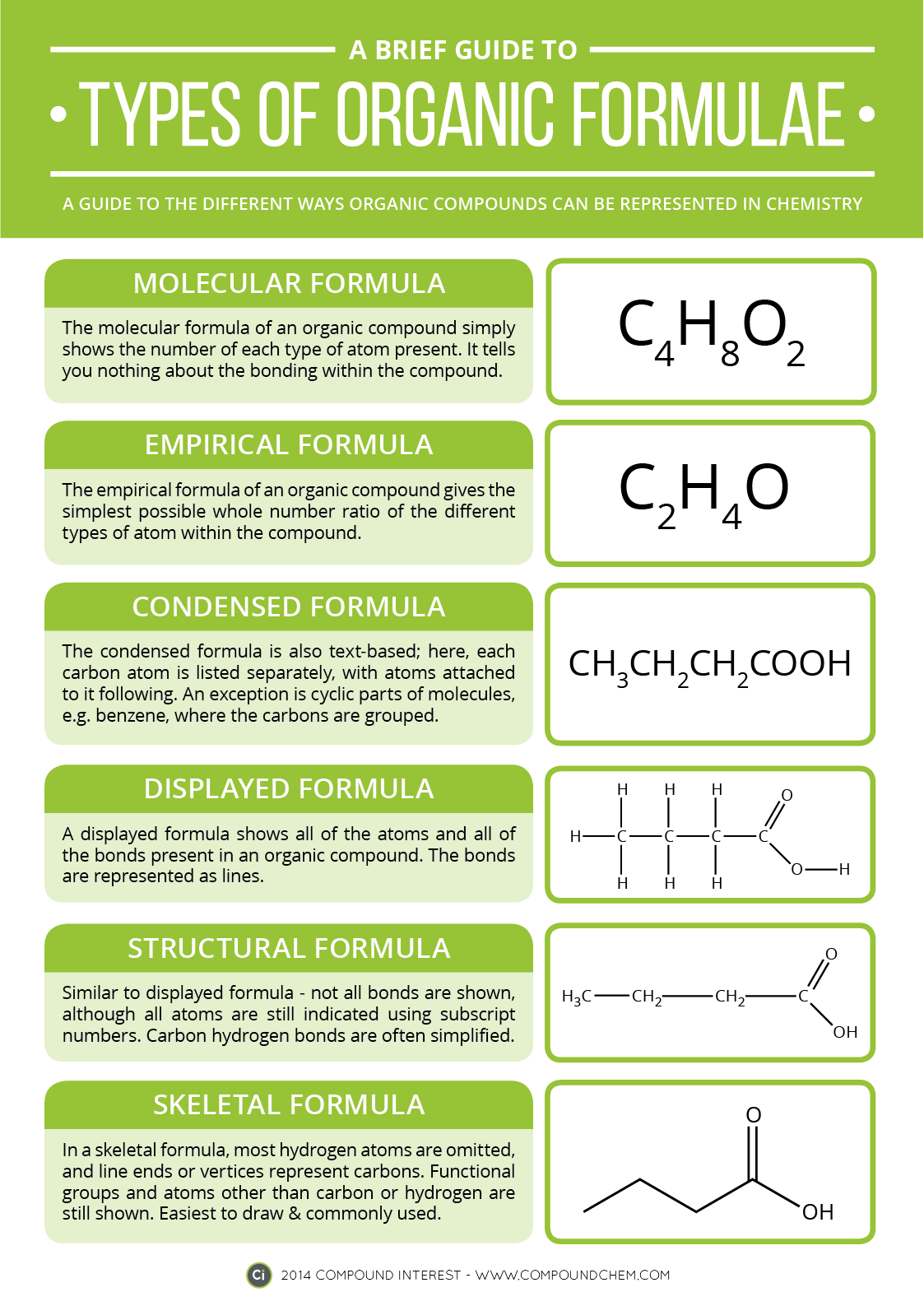
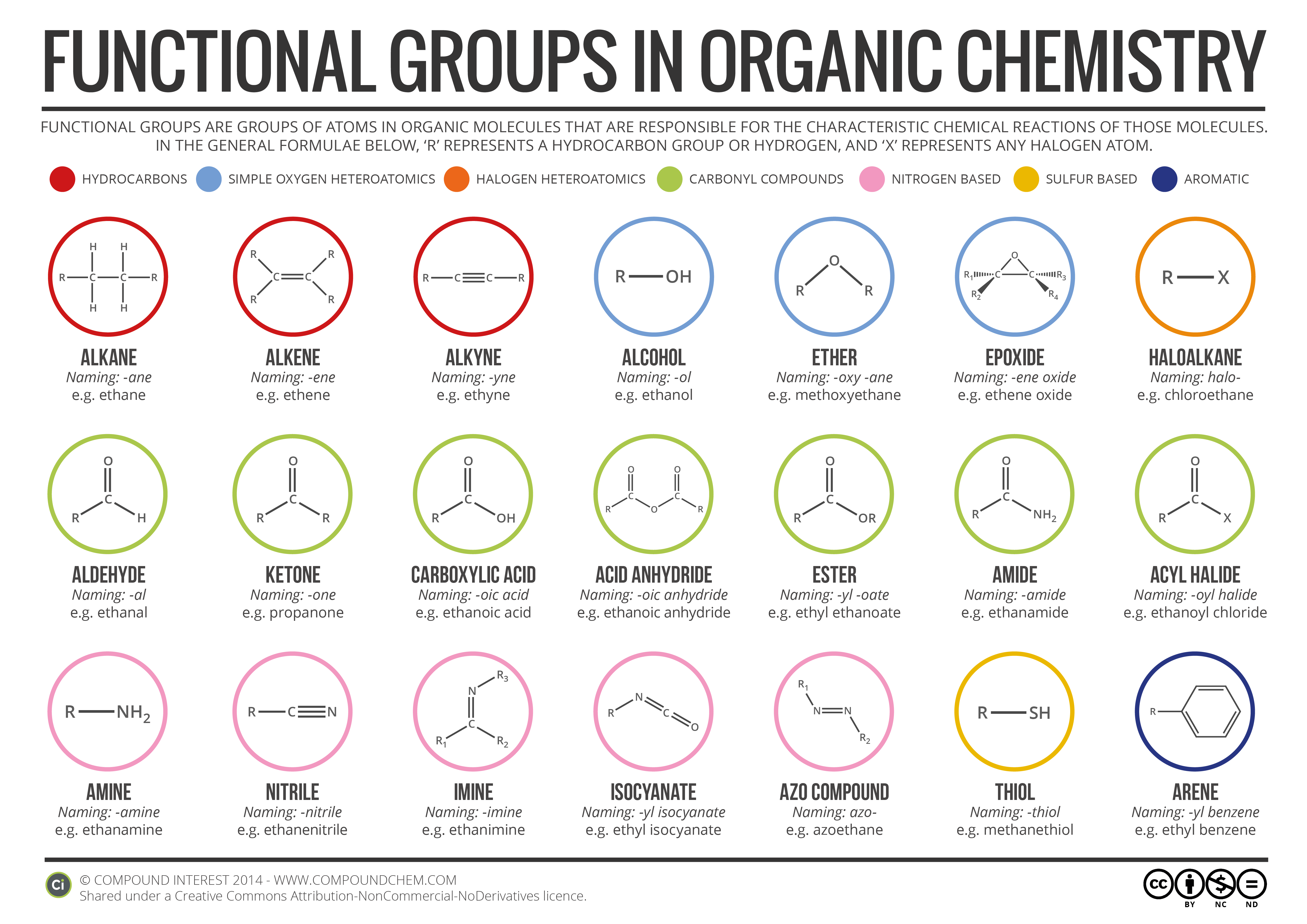
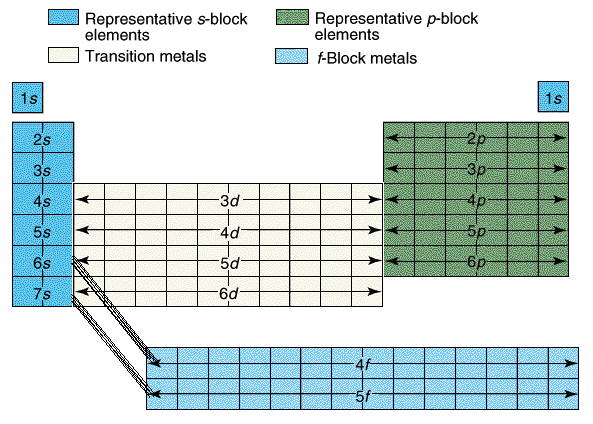
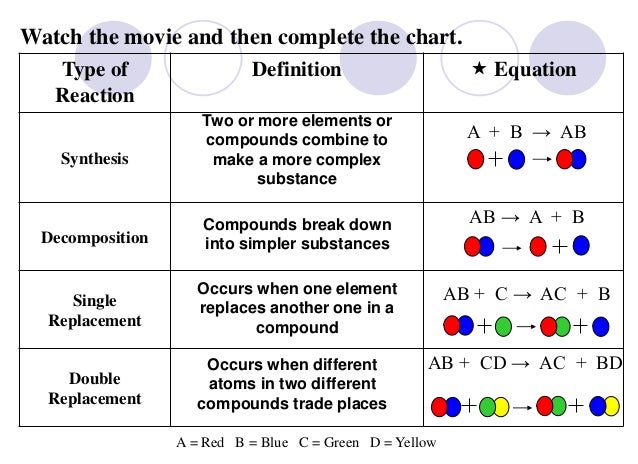
 We started by talking about the simple definition of the terms, what the probably products and reactants are and went over a basic formula for the reaction types the students need to be familiar with.
We started by talking about the simple definition of the terms, what the probably products and reactants are and went over a basic formula for the reaction types the students need to be familiar with.Reaction Types include:
- synthesis
- decomposition
- singe replacement
- double replacement
- combustion
- endothermic
- exothermic
- oxidation-reduction
- neutralization
- nuclear
Can you guess what type this is?