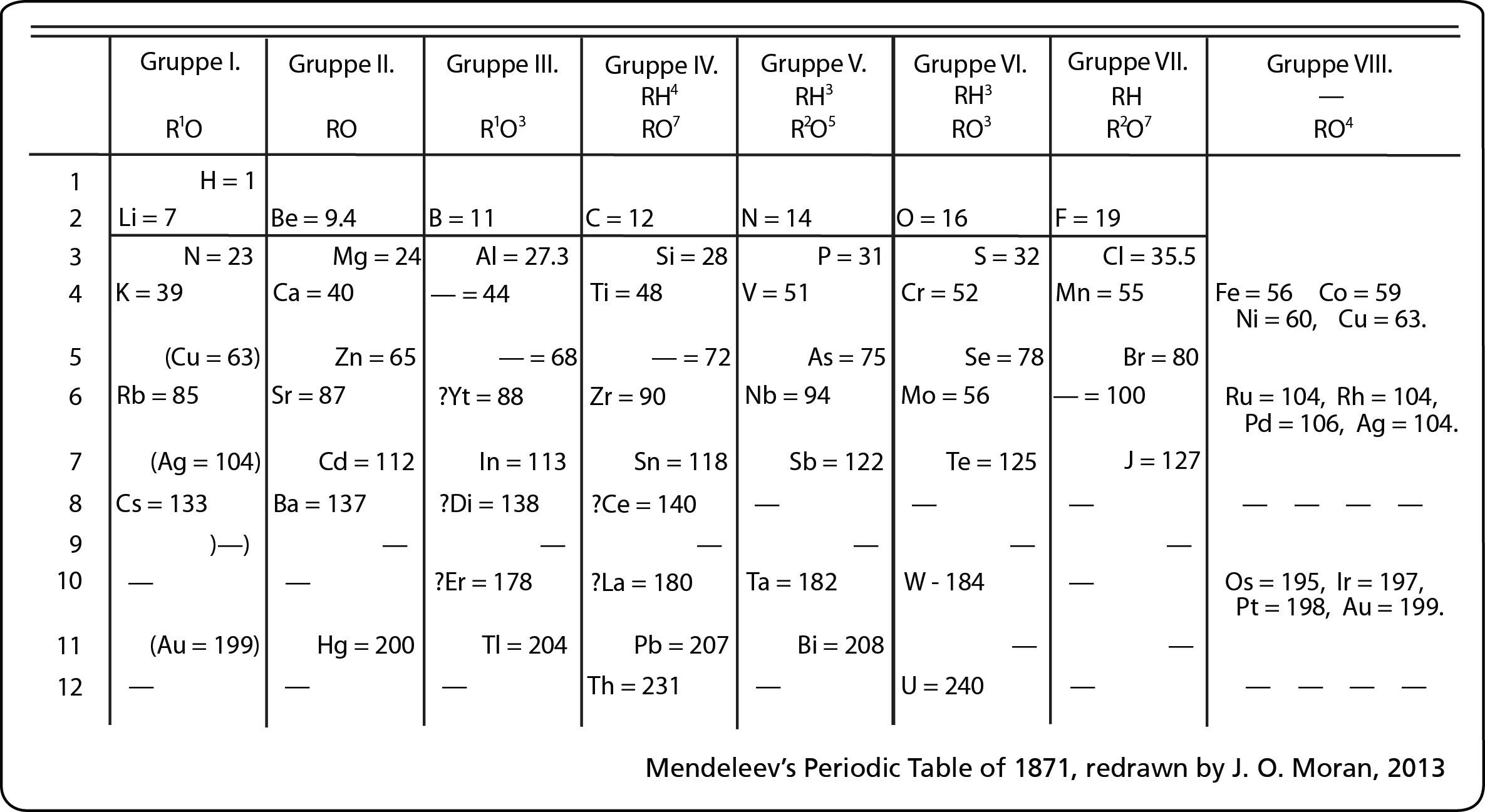
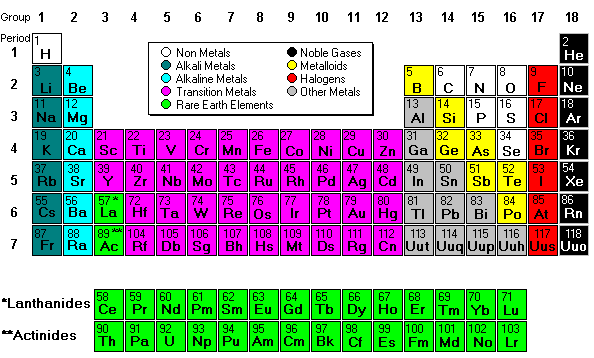
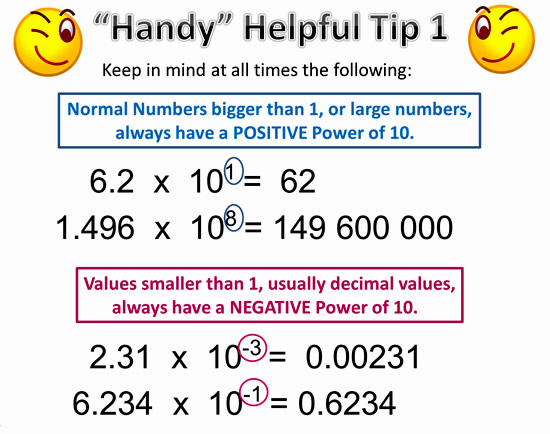
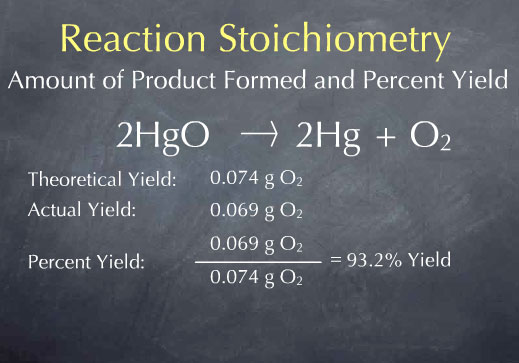
Heisenberg and De Broglie came up with the current electron cloud model, but we draw Bohr's planetary model the most often because it easier to count the electrons. There's a TedEd video about the progression - HERE.
Electrons are tricky because they move constantly and at high speeds. Heisenberg's Uncertainty Principle states that you cannot know both the speed and location of an electron - you can only know one - because measuring either one, changes the other. DeBroglie's wave theory helps explain why electrons sometimes act like particles and sometimes are compared to waves.
For more information about the evolution of the atomic model, check out this link. And here is a video!
Here are some Dude Quizlet Flashcards to help you out.