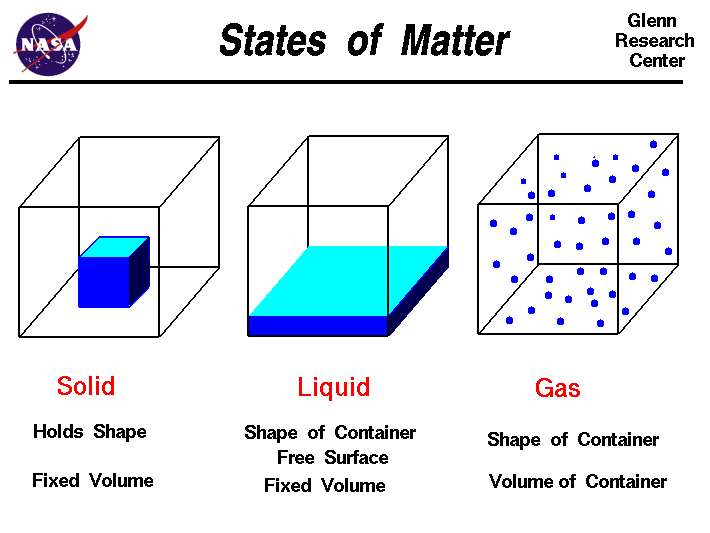
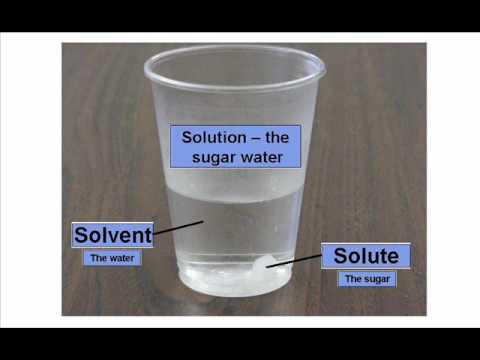
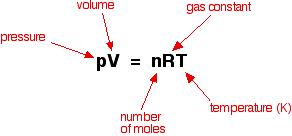
Greetings Chemistry students!
You should have your Unit 8 Notepacket and a handout from Mrs. Windsor... otherwise how did you get to this webpage?
General rules for the computer lab - do your chemistry work. This is not time to work on stuff for other classes, check your email, or anything else. So don't. Do chemistry.
First, you are going to download a powerpoint on acids and bases and take notes. Download it HERE! You can save it if you want, otherwise just choose open.(For GovSchool students on Friday's field trip, you also need these notes and to get a lab from Mrs. Windsor. You need to do as much as possible on that sheet.)
Once you are done with the notes, you are going to read through the interactive instructions on the handout from Mrs. Windsor. Then click HERE to go to the interactive and do it! (Alkali = base!)
Once you are done with the interactive, you are going to go take the Radiation Quiz and answer the questions. We live at 500 feet. (This is the one I breezed through recently.) We will be talking about the nuclear particles and reactions on Monday.
Then get your homework (it's pink!) from Mrs. Windsor and get started. She will be checking it and going over it tomorrow.
- There will be an acid base quiz on Monday.
- Tuesday = Benchmark 3
- Friday = Chemistry Assessment (you took at beginning of year)
- Wednesday the 12th = Chemistry SOL