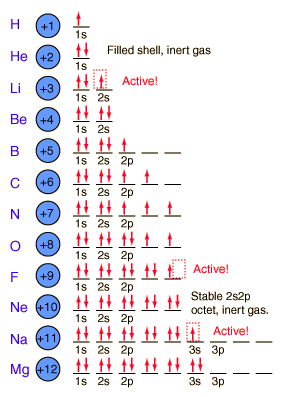
Today students started with a jump in where they identified and counted parts of an atom on drawings. Once they figured out the atomic number (number of protons), they could then tell which atom on the periodic table it was supposed to be.
Students were given a little sample of 5 paint chips and asked to sort them. Most sorted them from lightest to darkest, which was fairly simple because all the paint chips were similar colors. Next students were given a sample with 12 different paint chips and asked to arrange them. Some arranged them in a long line while others put them in groups. Eventually students were asked to put them in three columns of four. Why?
Mendeleev deisgned the periodic table by looking at the properties of elements on cards and arranging them different ways until he got a system that worked. No one told him how to do it, he just did it until it worked. He even left spaces for elements that were discovered in his lifetime. (
More info about Mendeleev) His periodic table was set up according to atomic mass number. The current table, altered slightly by Moseley, is organized by atomic number (number of protons).
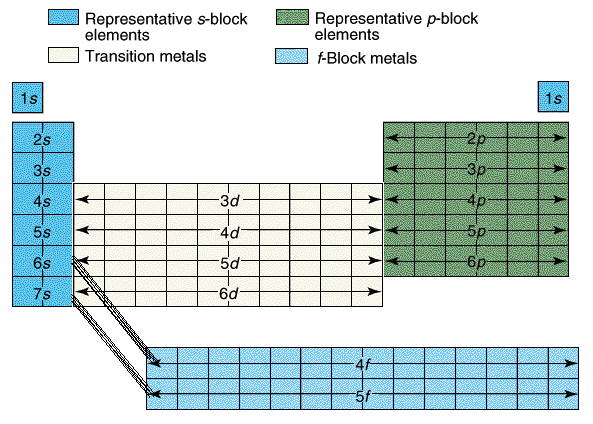
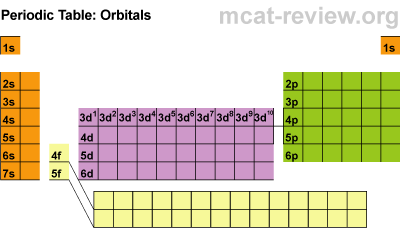
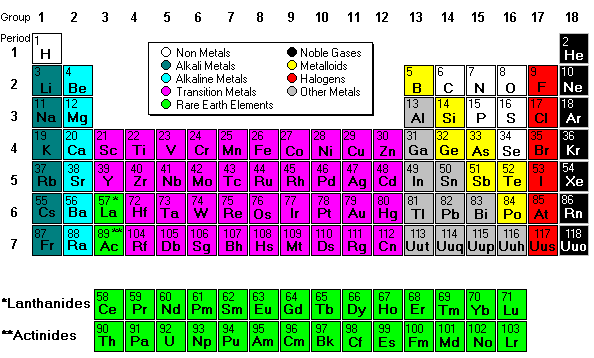
Next we discussed regions of the periodic table, colored them, and labeled them. Periods are horizontal rows (periods go at the end of a sentence) and there are 7 periods. There are 18 groups or families (vertical columns) and a few of them have special names. This a pretty excellent diagram.
This website gives a lot of helpful information.
In third period, we finished class by playing Guess Who with the Periodic Table in partners. It is a great way to practice naming and identifying the various regions. Some of them were really getting into it which makes the game more interesting. As the students learn the terms they will play against each other instead of asking questions about the element that I have picked.

Fourth period finished the day with the Physical Chemical Lab from last week (we were interrupted by a fire drill). Many students were able to use the time to finish up the questions on the back as well. Fourth will have opportunities to play Guess Who later this week.
HOMEWORK - Atom Math
Physical/Chemical Lab due Friday

We also talked in class about other ways to arrange periodic tables and I found these neat examples. I also like this idea -
a periodic table of imaginary elements made up from made-up elements from movies and books - like Superman and Star Trek.