Students made a foldable reviewing all the dudes they need to know that influenced the development of the atomic model. Students tried it out without their notes and realized how little they remembered... and then used their notes to record the information. We also watched a BrainPop about the atomic models to help refresh their memories. Now that they have their foldables made, they can use them to study for the unit test and to study for the SOL.
For more information about the evolution of the atomic model, check out this link.
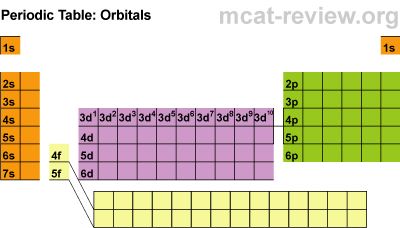
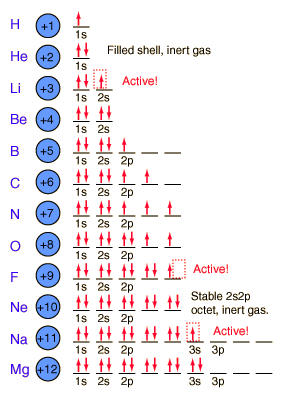
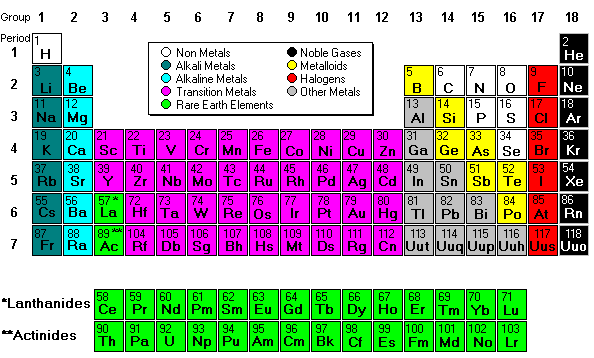
Next we finished up the notes by discussing periodic trends.
Electro- negativity is how badly atoms want electrons. The most electronegative atoms are Fluorine, Chlorine, and Oxygen. Ionization energy is how difficult it is to remove electrons. It is difficult to remove electrons from atoms that are electronegative.
Atomic radius increases as you move down the periodic table because atoms have more mass, but actually decreases from left to right because atoms are holding on to their electrons tighter (because they are more electronegative).
Global Ocean Conveyor
-
Today students began class with a BrainPop on currents and took the quiz.
Tim and Moby did an excellent job describing how currents affect global
weather ...
6 years ago