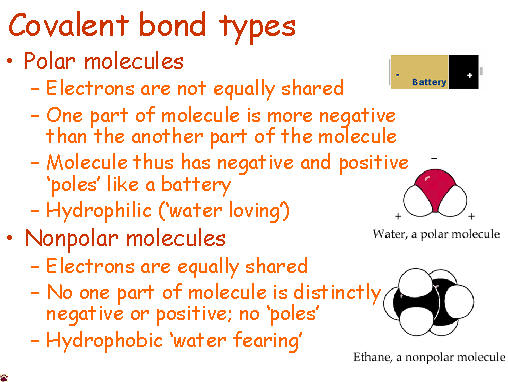
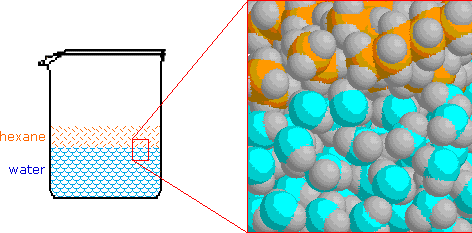
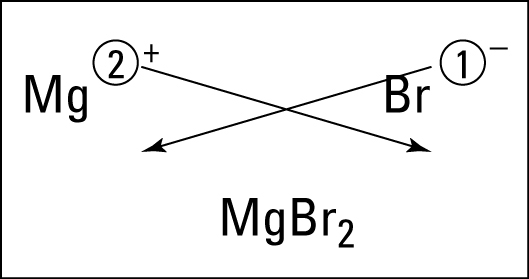
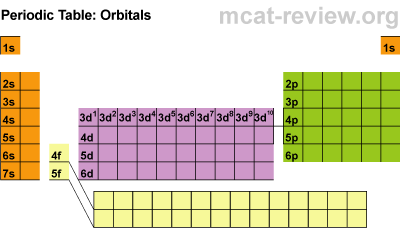
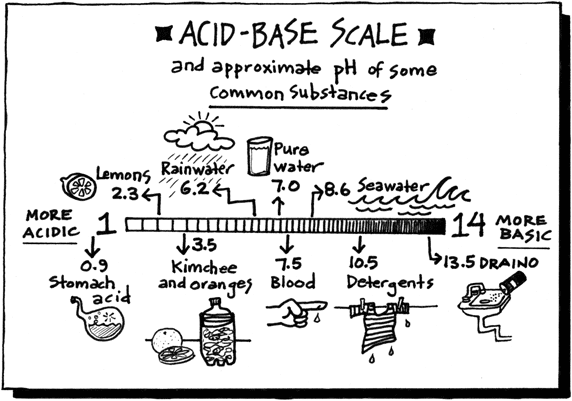
Today, we started talking about moles. Moles are used to count atoms. There are 22,000,000,000,000,000,000 quintillion atoms in a grain of sand and even counting grains of sand is a pain. Because atoms are so tiny, we use the mole to estimate.
There are 6.02 x 10 ^23 molecules in one mole. That's a whole lot. This is our new favorite number because it needs to be memorized. We practiced converting from moles to molecules.
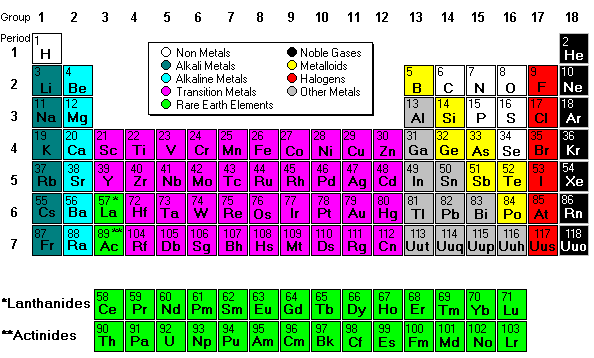
Next we discussed molar mass. Molar mass = 1 mole and it also equals atomic mass from the periodic table. To find the molar mass of carbon dioxide you find the mass of carbon and two oxygens and add them together. Finding molar mass is not difficult unless the molecule has tricky subscripts (which we have been practicing).
Global Ocean Conveyor
-
Today students began class with a BrainPop on currents and took the quiz.
Tim and Moby did an excellent job describing how currents affect global
weather ...
6 years ago