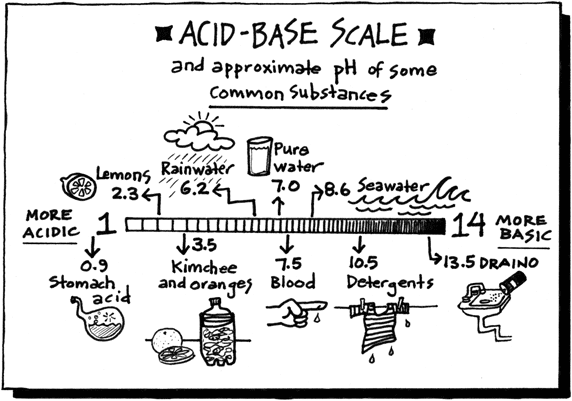
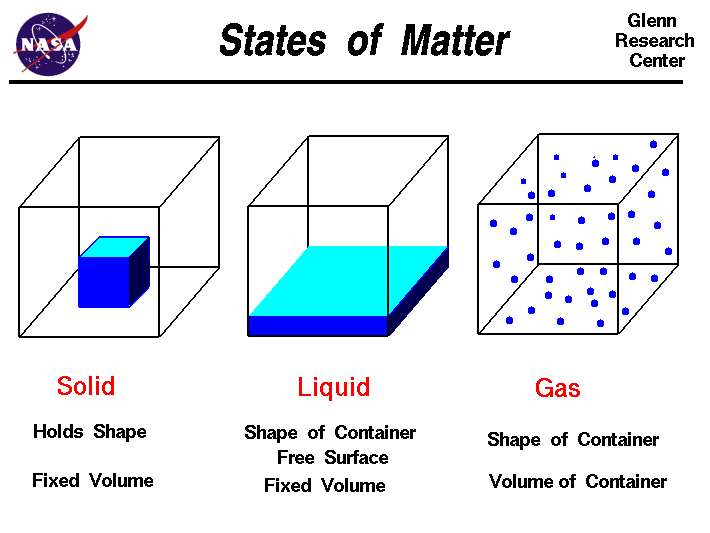
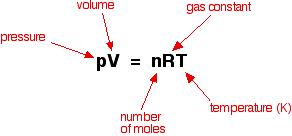
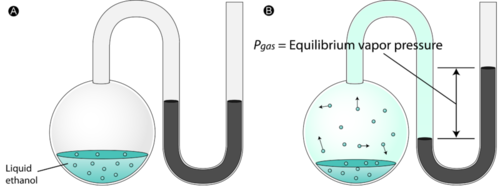
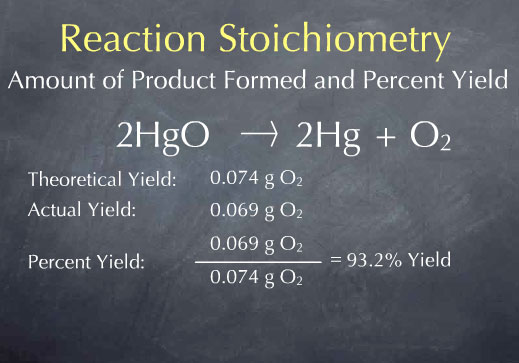
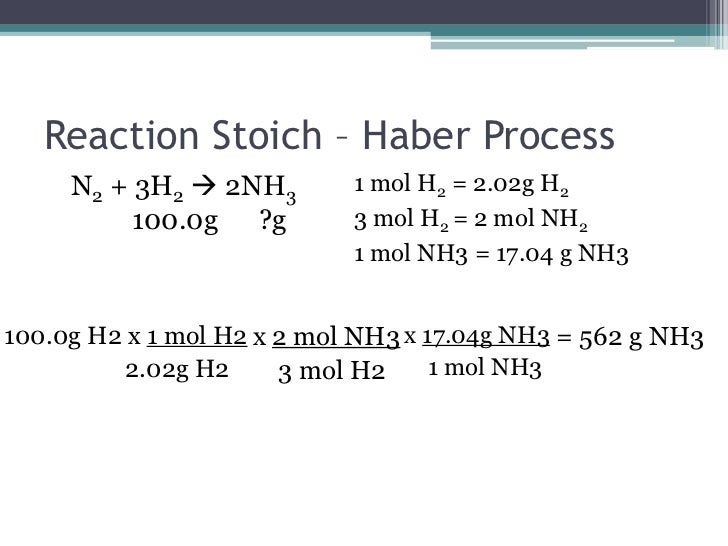
Acids have H+ and donate them, they have the smaller numbers 0-6.9, they turn litmus red, and have sour tastes.
Bases
have OH- and want H+ to make water, they have Bigger numbers, they have
a pH between 7.1 and 14, they turn litmus Blue, and they taste Bitter.
If you mix an acid and a base together, then there is a neutralization reaction. A neutralization reaction is also a double replacement reaction. In neutralization, the acid and base combine to form a water and a salt. The water and the salt are neutral (hence the name).
pH measures the concentration or molarity of H (Hydrogen ions) in a solution. That's why the H is capitalized.
pH + pOH = 14. So if you have the pH, it is easy to get the pOH... just subtract from 14. There are some fun interactives for acids and bases linked at the bottom of this webpage. Check out the Alien Juice Bar.