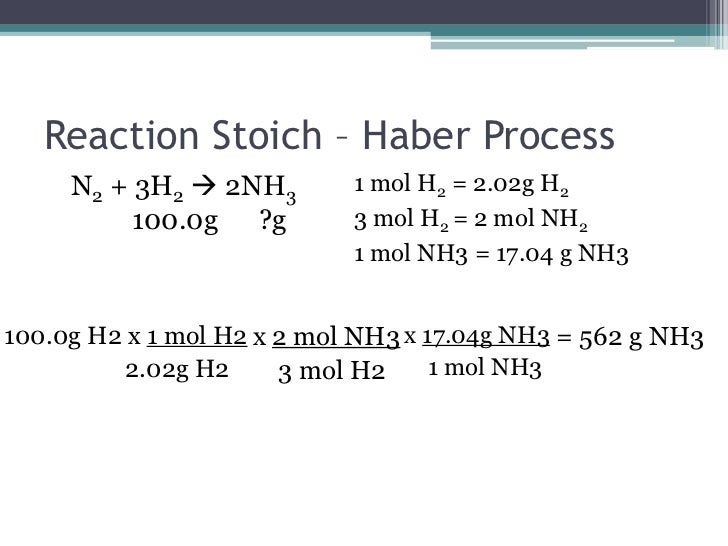
Stoichiometry uses the three mole conversions that students are familiar with from unit one, plus the mole/mole conversion. A mole/mole conversion uses the coefficients from a balanced equation to convert from one chemical to another. You can only compare elements or chemicals when they are both in mole form.
Using this equation N2 + 3 H2 --> 2 NH3 the following calculations can be made using stoichiometry.
Check out this video from CrashCourse if you need some help!









No comments:
Post a Comment