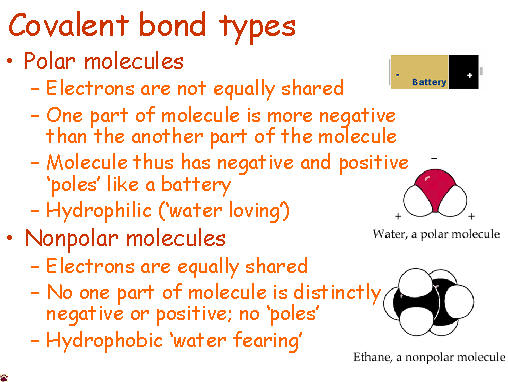
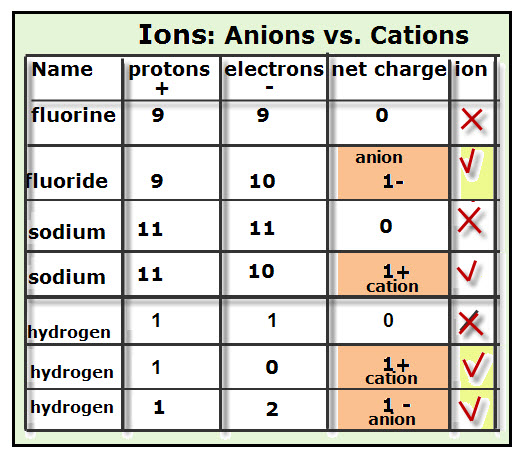
Today after a jump in and going over last night's homework, students took a short quiz on these topics (Valence electrons, finding charges, using Roman numerals, counting atoms). I have a feeling they did pretty well :)

Students then learned about ionic bonding. Ionic bonding happens between metals & nonmetals (positives & negatives). The electrons are given and taken in this ionic bond. To get the formula, you criss cross the charges. To name it, you say the name of the metal, then the name of the nonmetal with an -ide ending. If it is a metal from DForP block, then you use a roman numeral to indicate the charge of the metal.
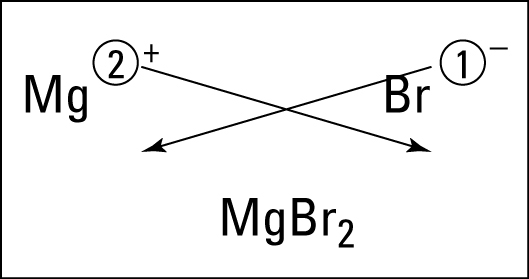
After learning the basics, students in first period practiced with an activity called "speed dating." Students were metals ("boys") and nonmetals ("girls") and practiced dating, bonding, and naming the ionic bonds they would make with their partners. The funny thing is that being a male did not necessarily make your character a "boy." :) Students really got the hang of bonding, were able to work with and help a variety of partners, and had fun. We will continue this activity tomorrow in all class periods.

Now that we understand ionic bonding, students should find this cartoon amusing.
Ionic Bonds for Dummies
Here is a cool interactive where you can build models to simulate ionic bonding.