Upwelling
-
Today in class we discussed water temperature and upwelling. For notes we
continued talking about water movement and addressed ocean temperature and
locat...
8 years ago
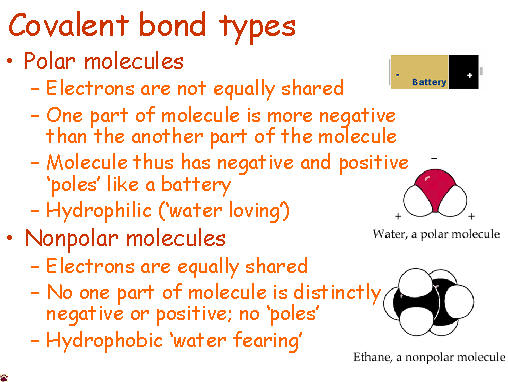 Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different.
Anyone who has ever had to share something with someone else knows that sometimes isn't exactly even. Covalent molecules or bonds are no different.  Time to learn about roman numerals.... Here is a handy clock if you are unfamiliar with them. Pretty much you need to know 1-7. 1 is represented with I, five with V and 10 with X. 4 and 6 and 7 is where it gets tricky. 4 is 1 before 5 - so its Roman numeral is IV. 6 is one after five so its roman numeral is VI.
Time to learn about roman numerals.... Here is a handy clock if you are unfamiliar with them. Pretty much you need to know 1-7. 1 is represented with I, five with V and 10 with X. 4 and 6 and 7 is where it gets tricky. 4 is 1 before 5 - so its Roman numeral is IV. 6 is one after five so its roman numeral is VI. Students learned about ionic bonding. Ionic bonding happens between metals and nonmetals (positives and negatives). The electrons are given and taken in this ionic bond.
Students learned about ionic bonding. Ionic bonding happens between metals and nonmetals (positives and negatives). The electrons are given and taken in this ionic bond. 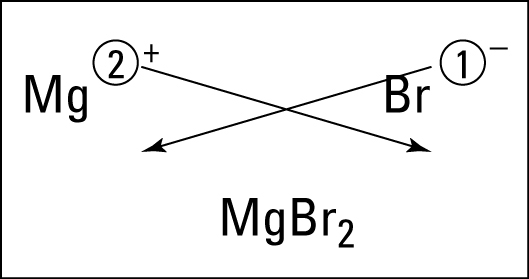 After learning the basics, students practice.
After learning the basics, students practice. Now that we understand ionic bonding, students should find this cartoon amusing.
Now that we understand ionic bonding, students should find this cartoon amusing.
- Ions are atoms or molecules that have a net charge, either positive or negative. There are two kinds of ions:
- Anions are negatively charged ions because they have negative net charges. This means that there is a greater number of electrons (-) than protons (+). For example, the anion, fluoride (F 1-), has a one negative charge because it has a total of nine protons and ten electrons. Thus, the net charge for fluoride is 1 negative.
- Cations are positively charged ions because they have positive net charges. This is due to these ions having more protons (positive charges) than electrons (negative charges). For example, calcium (Ca 2+) is a cation ion with 20 protons and 18 electrons. The net charge for Calcium is 2 positive. (from here)
 Today we discussed how to write chemical formulas, and what the numbers associated with a chemical formula mean.
Today we discussed how to write chemical formulas, and what the numbers associated with a chemical formula mean.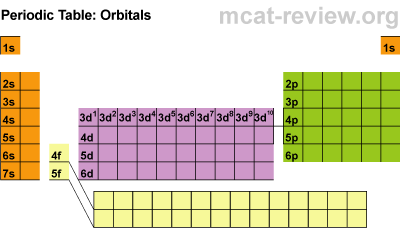 Battleship, a classic game by Milton Bradley, is a game easily adaptable to learning electronic configuration.
Battleship, a classic game by Milton Bradley, is a game easily adaptable to learning electronic configuration.
