Upwelling
-
Today in class we discussed water temperature and upwelling. For notes we
continued talking about water movement and addressed ocean temperature and
locat...
8 years ago
 Pressure
is the tricky one. If pressure is applied to an equilibrium, then the
reaction will shift to the side that has the least amount of molecules
(count the coefficients).
Pressure
is the tricky one. If pressure is applied to an equilibrium, then the
reaction will shift to the side that has the least amount of molecules
(count the coefficients). Reaction
Rates are affected by a few things. Without telling them the point, the
students had a quick demo where they had to dissolve sugar cubes the
fastest. The things that speed up reactions are:
Reaction
Rates are affected by a few things. Without telling them the point, the
students had a quick demo where they had to dissolve sugar cubes the
fastest. The things that speed up reactions are: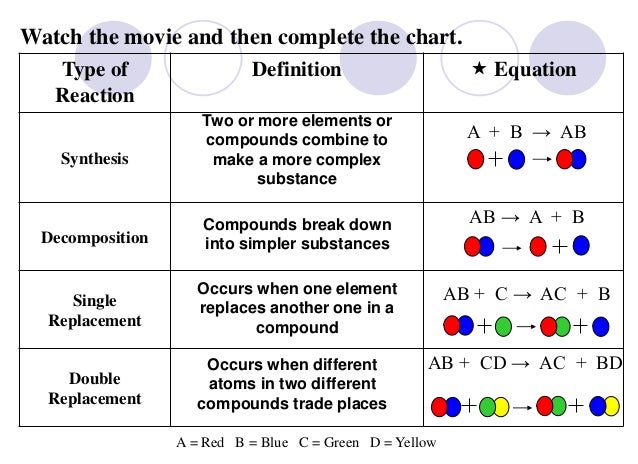 We
started by talking about the simple definition of the terms, what the
probably products and reactants are and went over a basic formula for
the reaction types the students need to be familiar with.
We
started by talking about the simple definition of the terms, what the
probably products and reactants are and went over a basic formula for
the reaction types the students need to be familiar with.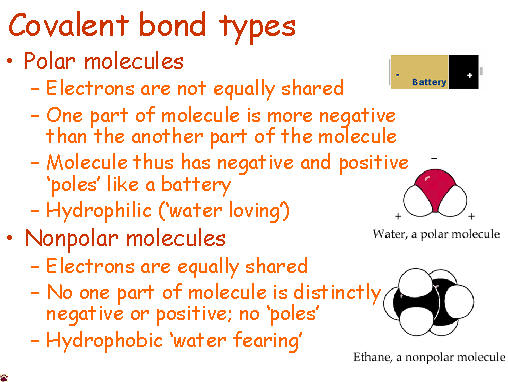 Anyone
who has ever had to share something with someone else knows that
sometimes isn't exactly even. Covalent molecules or bonds are no
different.
Anyone
who has ever had to share something with someone else knows that
sometimes isn't exactly even. Covalent molecules or bonds are no
different. 
