 Today we started class by going over yesterday's density problem homework and the physical and chemical homework from the day before. I brought up the density triangle that they may have learned in eighth grade to help them solve difficult problems.
Today we started class by going over yesterday's density problem homework and the physical and chemical homework from the day before. I brought up the density triangle that they may have learned in eighth grade to help them solve difficult problems.Next students worked on sorting characteristics of solids, liquids, and gases. Then students worked with matching cards on a variety of current topics like physical and chemical changes, phase changes, phases, and types of matter like element and compounds. Students seemed to enjoy matching the cards and some teams were racing. I think it was helpful for them to review sometimes confusing and similar concepts.
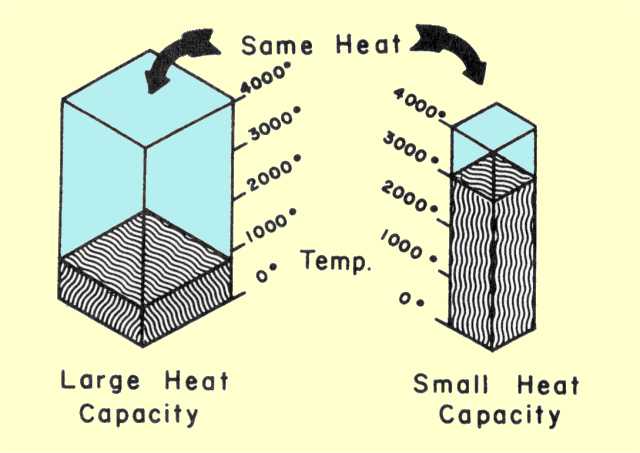 We did a little bit of notes about specific heat and heat capacity. Heat capacity is the amount of energy needed to raise a substance 1*C. Heat capacities depend on the amount of the liquid and how it is contained.
We did a little bit of notes about specific heat and heat capacity. Heat capacity is the amount of energy needed to raise a substance 1*C. Heat capacities depend on the amount of the liquid and how it is contained.Specific heat is the amount of heat needed to raise 1 gram of a substance 1*C. Specific heat is measured using a formula with J/gC. Apparently that now stands for Jancaitis gone crazy instead of Joules per grams Celsius.








No comments:
Post a Comment